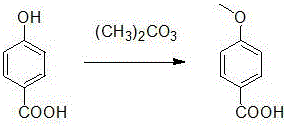Method for synthesizing p-methoxybenzoic acid
A technology of methoxybenzoic acid and p-hydroxybenzoic acid, which is applied in the field of industrial synthesis of p-methoxybenzoic acid, can solve the problems of high reaction temperature, extended production cycle, and high preparation cost, and achieve high product yield and safety Good performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
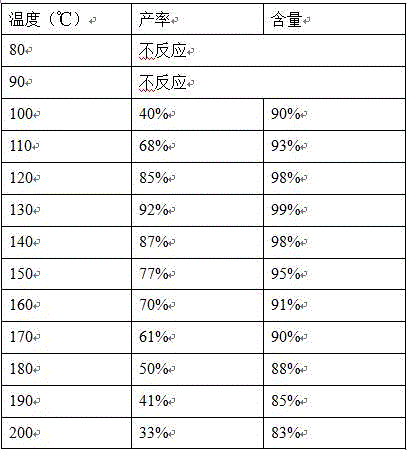
[0026] Dissolve 13.8g of p-hydroxybenzoic acid, 13.5g of dimethyl carbonate, and 0.16g of tetrabutylammonium bromide in 150ml of methanol. After dissolving, pump it into the microreactor with a flow rate of 3mL / min and a reaction pressure of 5MPa. Heat to 120°C, residence time 20min, after about 1.5 hours, the reaction was completed, part of the methanol was recovered from the feed liquid, the feed liquid was cooled to room temperature, filtered, and dried to obtain 12.9 g of a white solid with a yield of 85% and a purity of 98%.
Embodiment 2
[0028] Dissolve 13.8g of p-hydroxybenzoic acid, 13.5g of dimethyl carbonate, and 0.16g of tetrabutylammonium bromide in 150ml of methanol. After dissolving, pump it into the microreactor with a flow rate of 3mL / min and a reaction pressure of 5MPa. Heat to 130°C, residence time 20min, the reaction ended after about 1.5 hours, part of the methanol was recovered from the feed liquid, the feed liquid was cooled to room temperature, filtered, and dried to obtain 14.0 g of white solid with a yield of 92% and a purity of 99%.
Embodiment 3
[0030] Dissolve 13.8g of p-hydroxybenzoic acid, 13.5g of dimethyl carbonate, and 0.16g of tetrabutylammonium bromide in 150ml of methanol. After dissolving, pump it into the microreactor with a flow rate of 3mL / min and a reaction pressure of 5MPa. Heat to 140°C, residence time 20min, after about 1.5 hours, the reaction was completed, part of the methanol was recovered from the feed liquid, the feed liquid was cooled to room temperature, filtered, and dried to obtain 13.2 g of white solid with a yield of 87% and a purity of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com