Parthenolide derivative, and medicinal composition, preparation method and use thereof
A technology of drugs and uses, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as drug resistance and insensitivity of tumor stem cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
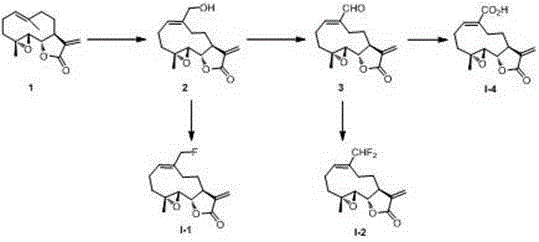
[0024] Mode( I ) in mono- and di-fluoro substituted parthenolide derivatives I-1 , I-2 and I-4 preparation of
[0025]
[0026] compound 2 Synthesis of: parthenolide (1.0 g, 4.3 mmol), SeO 2 (324 mg, 2.4 mmol) with Na 2 SO 4 pre-dried t -BuOOH ( 70% in H 2 O, 1.48 mL, 10.8 mmol) in dichloromethane (20 mL) at room temperature for 4 days, concentrated under reduced pressure, silica gel column chromatography (petroleum ether: ethyl acetate = 1:2), and obtained 810 mg of white solid , Yield: 72%. mp 171–173 o C; [α] D 21 –33.9 o ( c 1.0, CHCl 3 ); IR (KBr) 3466, 3096, 2957, 2867, 1747, 1309, 1151, 818 cm – 1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 6.20 (d, J = 3.4 Hz, 1H), 5.63 (t, J = 8.1 Hz, 1H), 5.54 (d, J = 3.1 Hz, 1H), 4.14 (d, J = 12.7 Hz, 1H), 4.05 (d, J = 12.7 Hz, 1H), 3.84 (t, J = 9.3 Hz, 1H), 2.84 (d, J = 9.4 Hz, 1H), 2.81 (m, 1H), 2.47–2.35 (m, 3H), 2.31–2.24 (m, 1H), 2.20–2.10 (m, 2H), 1.98 (br s, 1H), 1.63 (t, J = 11.2 Hz,...
Embodiment 2
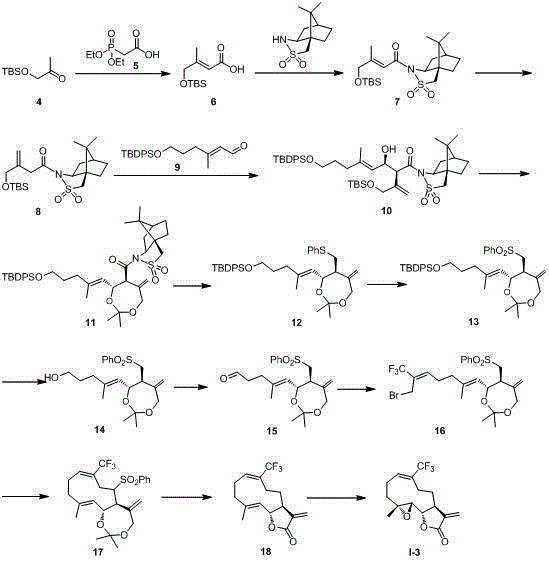
[0032] Mode( I ) in trifluoromethylated parthenolide derivatives I-3 preparation of
[0033]
[0034] compound 6 The synthesis of: under the protection of argon, the compound 5 (12.34 g, 62.93 mmol) was dissolved in dry tetrahydrofuran (125 mL), and NaHMDS (2 M in THF, 63.0 mL, 126.0 mmol) was added in an ice-water bath. After stirring for half an hour, dry tetrahydrofuran (250 mL) was added . Then the compound 4 (14.23 g, 75.53 mmol) was slowly added dropwise. Stirred under ice-water bath for 4 h, with KHSO 4 The solution (0.5 M) was quenched, and the pH of the solution was adjusted to 4, extracted, concentrated under reduced pressure, and silica gel column chromatography (petroleum ether: ethyl acetate = 20:1-8:1) to obtain the compound 6 (11.67 g, 80%). Colorless oil, IR (KBr) 3069, 2986, 2930, 2856, 1587, 1470, 1308, 1217, 1110, 704 cm – 1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 11.70 (s, 1H), 5.95 (s, 1H), 4.05 (s, 2H), 1.97 (s, 3H), 0.84 (s, 9H), 0.00 (s...
Embodiment 3
[0047] Example 3 : Pharmacological effects of parthenolide derivatives
[0048] Match various cancer cells into 2×10 5 / mL cell suspension, add to 24-well round-bottomed cell culture plate, add woody hydrocarbon lactone derivatives or their salts respectively, 5 wells for each test concentration, place at 37 ℃, 5% CO 2 After culturing under saturated humidity conditions for 18 hours, the absorbance (A) value was measured at a wavelength of 570 nm in an enzyme-linked detector by the MTT method, and the inhibitory effect of the compound of the present invention on the test cancer cells was calculated.
[0049] Table 1 Inhibitory activity of parthenolide derivatives on various cancer cells (IC 50 , μM)
[0050] [0056] cell
[0057] Compound I-1
[0058] Compound I-2
[0059] Compound I-3
[0060] Compound I-4
[0061] HL-60
[0062] 2.0
[0063] 2.0
[0064] 2.1
[0065] 6.3
[0066] HL-60 / A
[0067] 1.5
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com