Digital PCR platform-based miRNA detection quality control kit and application method thereof
A technology for testing quality and kits, which is applied in the biological field, can solve the problems of greatly affecting the test results, and achieve the effects of avoiding PCR false positive amplification, simple operation, and not easy to degrade
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: a kind of miRNA detection quality control kit based on digital PCR platform, it comprises artificially synthesized miRNA, reverse transcription primer, detects upper primer, detects lower primer and DEPC water, and described artificially synthesized miRNA primer sequence is as SEQID As shown in No.1, the reverse transcription primer sequence is shown in SEQ ID No.2, the detection primer sequence is shown in SEQ ID No.3, and the detection primer sequence is shown in SEQ ID No.4 ;
[0028] The artificially synthesized miRNA concentration is 3×10 6 copies / μl, the volume is 8 μl, the concentration of the reverse transcription primer is 5 μM, and the volume is 1 μl; the concentration of the detection upper primer and the detection lower primer is 10 μM, each volume is 1 μl; the mass percentage concentration of the DEPC water It is 1%, and the volume is 1ml.
Embodiment 2
[0029] Embodiment 2: a kind of using method of miRNA detection quality control kit based on digital PCR platform, it comprises the following steps:
[0030] S1. Dilute the artificially synthesized miRNA to 1 / 10 times the original concentration with DEPC water;
[0031] S2. Extract the total miRNA of the biological body fluid as a sample, add lysate buffer to lyse the RNase in the sample, and add 4 μl of diluted artificially synthesized miRNA to the sample;
[0032] S3. Set the final dissolving volume of the total miRNA in the extracted sample to be 10-60 μl (volume A), and prepare a control sample. The specific steps are: draw 4 μl of diluted artificially synthesized miRNA and dissolve it in (A-4 μl) volume of DEPC water;
[0033] S4. While reverse-transcribing the target miRNA to be detected in the sample, reverse-transcribe the artificially synthesized miRNA in the sample and the control sample separately, and add 1 μl of reverse transcription primer to each 10 μL reaction s...
Embodiment 3
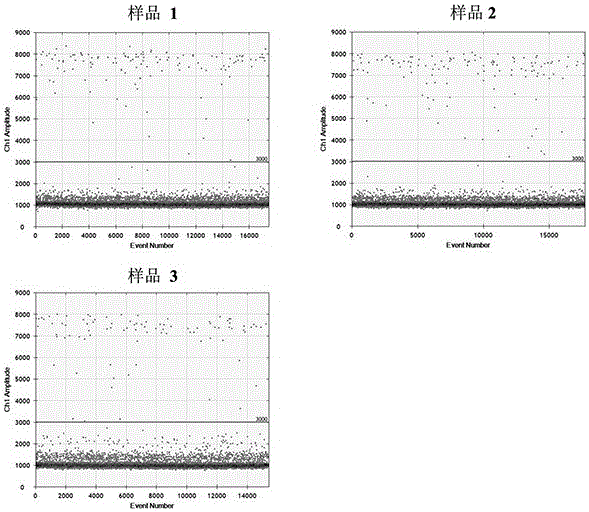
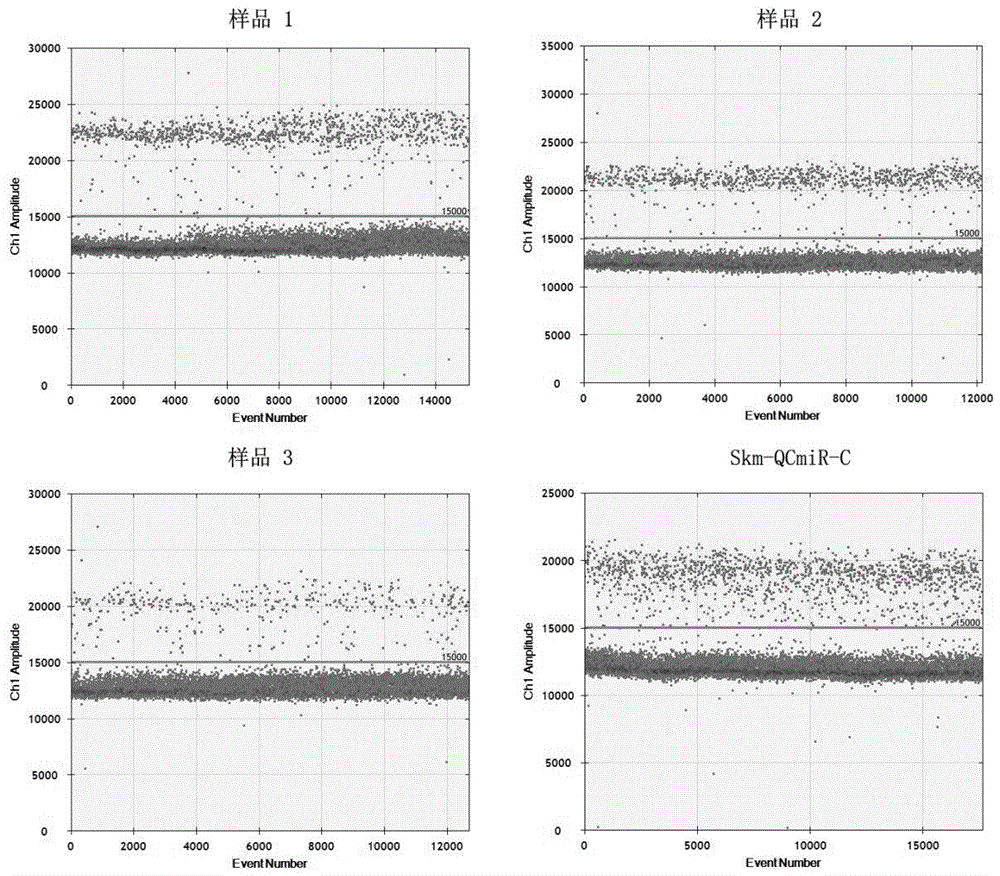
[0039] Example 3: Quality control in the process of detecting the expression level of miRNA-122 in healthy human plasma based on a digital PCR platform
[0040] 1. Sample collection and processing
[0041] Use Streck's Cell-Free DNA BCTTM tube to collect 5ml of blood from healthy people. Transfer the blood to a new 15ml centrifuge tube, centrifuge at 2000g for 10min at 4°C, and draw the upper layer of plasma into a new 15ml centrifuge tube. Then centrifuge the plasma at 4000g for 20min at 4°C, and draw the upper layer of plasma into a new 15ml centrifuge tube. Pipette 200 μL of plasma into a new 1.5ml EP tube, and use QIAGEN's miRNeasy Serum / Plasma Kit to extract total RNA. Among them, after adding QIAzolLysis Reagent and standing for 5 minutes, 4 μL of artificially synthesized miRNA (hereinafter referred to as Skm-QCmiR) diluted by 10 times in this kit was added, and the subsequent experimental operation was carried out according to the operation manual, and the final disso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com