Preparation method of peramivir powder
A technology of peramivir powder and mivir powder, which is applied in the field of preparation of peramivir powder, can solve the problems of uneven particle size, excessive particle size difference, triggering asthma, etc., and achieve uniform powder particle size and good effect Good, good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] A preparation method of peramivir powder, comprising steps:
[0018] A, take raw material peramivir;
[0019] B, dry the raw material peramivir taken in step A, grind, pass through 500 mesh sieve, 800 mesh sieve successively, get -500~+800 mesh particles to obtain dried peramivir;
[0020] C. Micronizing the dried raw material in step B in a jet mill to obtain a micronized powder;
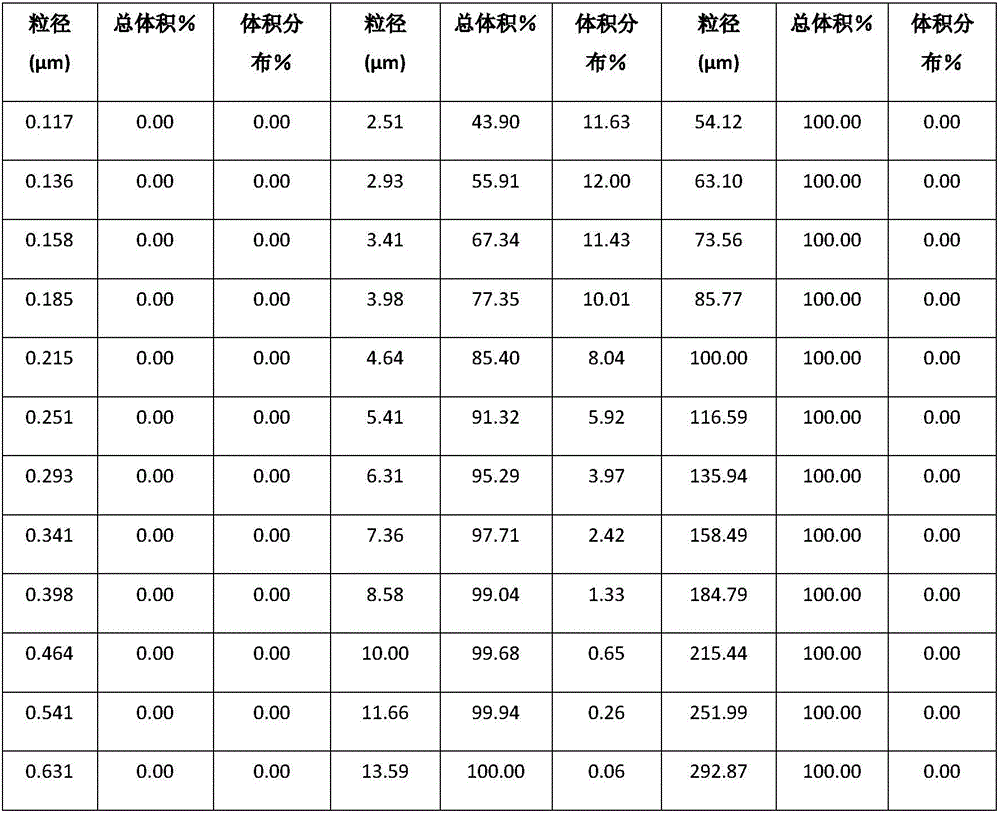
[0021] D. Pass the micronized powder obtained in step C through a 1000 mesh sieve to obtain micronized peramivir powder.
[0022] The particle size of the micronized peramivir powder obtained after passing the obtained micronized powder through a 1000-mesh sieve is within a reasonable range.
[0023] A further improvement is that the temperature of the drying treatment in the step B is 30-50° C., and the drying time is 12-24 hours.
[0024] A further improvement is that the temperature of the drying treatment in step B is 30° C., and the drying time is 24 hours.
[0025] A further improv...
Embodiment 1
[0030] A preparation method of peramivir powder, comprising steps:
[0031] A, take raw material peramivir;
[0032] B. Dry the peramivir raw material weighed in step A at 30°C for 24 hours, grind, pass through a 500-mesh sieve and an 800-mesh sieve in turn, and take -500 to +800 mesh granules to obtain dried peramivir ;
[0033] C. Micronize the peramivir obtained in step B in a jet mill, and set the pulverization particle size of the jet mill to 1.0-5.0 μm to obtain micronized peramivir powder.
[0034] D. Pass the micronized powder obtained in step C through a 1000 mesh sieve to obtain micronized peramivir powder.
Embodiment 2
[0036] A preparation method of peramivir powder, comprising steps:
[0037] A, take raw material peramivir;
[0038] B. Dry the peramivir raw material weighed in step A at 50°C for 12 hours, grind, pass through a 500-mesh sieve and an 800-mesh sieve in turn, and take -500 to +800 mesh granules to obtain dried peramivir ;
[0039] C. Micronize the peramivir obtained in step B in a jet mill, and set the pulverization particle size of the jet mill to 1.0-5.0 μm to obtain micronized peramivir powder.
[0040] D. Pass the micronized powder obtained in step C through a 1000 mesh sieve to obtain micronized peramivir powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Grinding particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com