Synthesis method of carbon nitride loaded copper catalyst
A technology of copper catalyst and synthesis method, applied in physical/chemical process catalysts, chemical instruments and methods, organic chemistry, etc., can solve problems such as unfavorable large-scale production, difficulty in recycling, and a large amount of waste, so as to improve activity and facilitate raw materials Obtained, the effect of simple process method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] 1. Combined with the catalyst:
[0013] Take 10mmol (1.26g) of melamine and 10mmol (1.88g) of copper nitrate into 20mL of water, stir evenly, then spread it on a watch glass, put it in an oven at about 60°C for 24 hours, take it out, and grind it into a powder. Using a tubular muffle furnace, in the air, the temperature was raised to 500°C at 5°C / min, calcined for 4h, taken out and cooled to obtain a nitrogen carbide supported copper catalyst.
[0014] 2. The activity of the obtained catalyst can be characterized by the coupling reaction between iodobenzene and 2-aminopyrimidine. The specific experimental steps are:
[0015] Take 5 mg of nitrogen carbide-supported copper catalyst and put it into the sealed tube, then add 142.65 mg of 2-aminopyrimidine (1.5 mmol) and 224.5 mg of potassium tert-butoxide (2 mmol), use the double-row tube to vacuumize and then fill with nitrogen, repeat this for 3 Second-rate. Under nitrogen gas, inject 204mg iodobenzene (1mmol) and 2ml d...
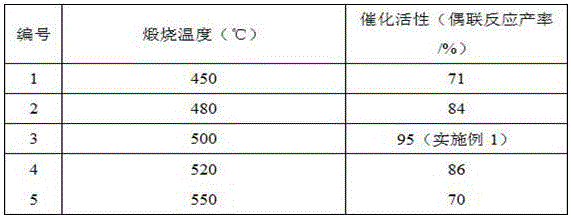
Embodiment 2
[0017] Embodiment 2: other conditions are the same as embodiment 1, and the activity of the catalyst prepared by different molar ratios of copper nitrate and ammonium cyanide is tested, and the experimental results are shown in Table 1.
[0018] Table 1 Test of the activity of catalysts prepared with different molar ratios of copper nitrate and ammonium trimer
[0019]
[0020] From the above results, it can be seen that the molar ratio of copper nitrate and cyanamide is preferably 100% (Example 1).
Embodiment 3
[0021] Embodiment 3: other conditions are the same as embodiment 1, and the activity of the catalyst prepared by different melamine and water consumption ratios is checked, and the experimental results are shown in Table 2.
[0022] Table 2 The test of the activity of the catalysts prepared by different ratios of triacylamine and water
[0023]
[0024] From the above results, it can be seen that the catalyst activity is the best when the ratio of triacylamine to water is 0.5 mol / L (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com