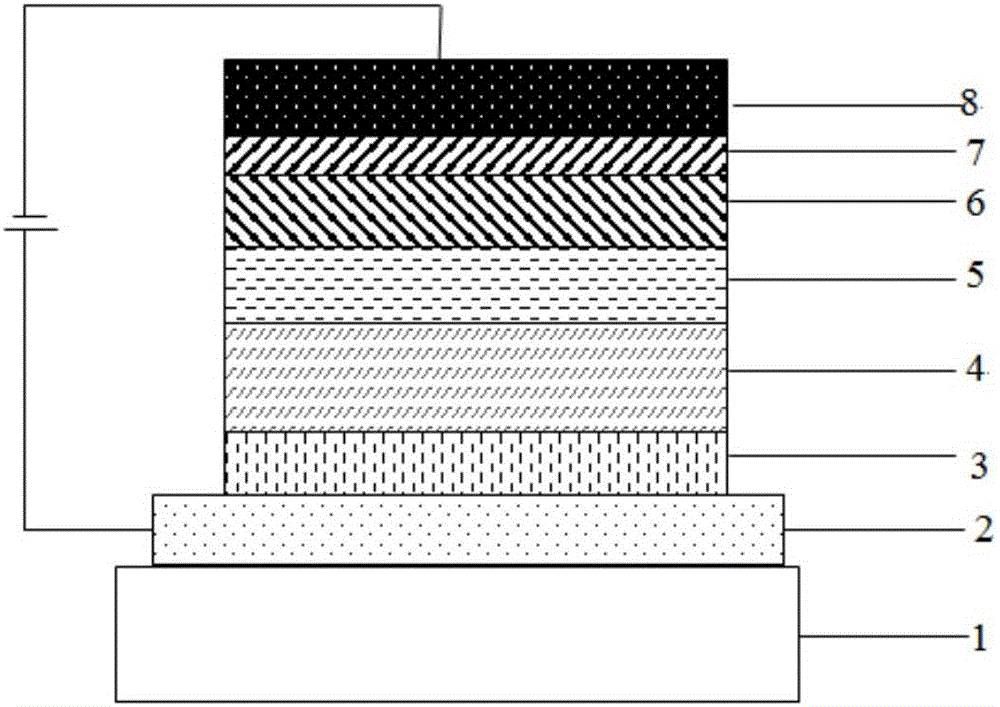
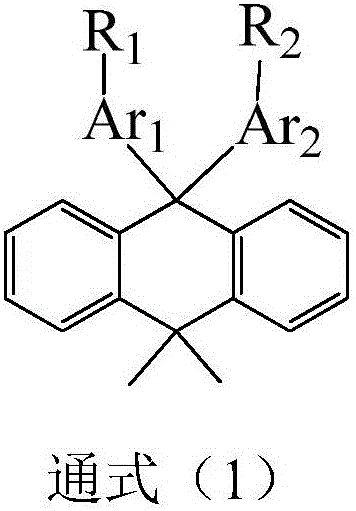
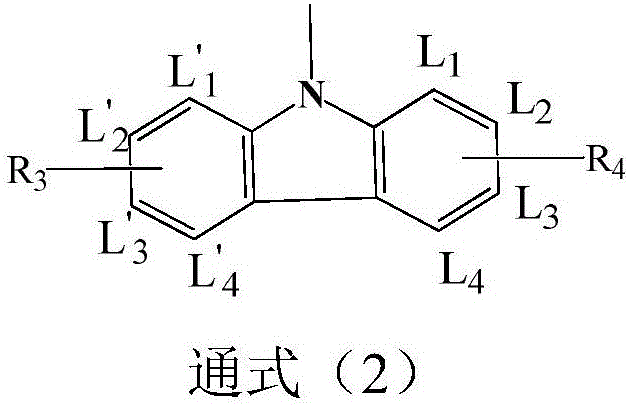
Dimethylanthracene-containing organic compound and application thereof
An organic compound, dimethyl anthracene technology, applied in the field of organic optoelectronic materials, can solve different problems such as avoiding aggregation, improving current efficiency and lifespan, improving exciton utilization rate and high fluorescence radiation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of intermediate A1:
[0058] synthetic route:
[0059]
[0060]
[0061] Add 11.8g of 1,4-dibromobenzene (0.05mol) and 1.33g of Mg powder (0.055mol) and 60ml of tetrahydrofuran to a 250ml four-necked flask under nitrogen atmosphere, heat and reflux for 4 hours, the reaction is complete, and the format Reagent;
[0062] Dissolve 11.1g of 10,10-dimethylanthrone (0.05mol) in 50ml of tetrahydrofuran, add the above-mentioned Grignard reagent dropwise, react at 60°C for 24 hours, a large amount of white precipitate is formed, and finally add saturated NHCl 4 Convert Grignard salt into alcohol; after the reaction, extract with ether, dry and rotary evaporate, petroleum ether: dichloromethane mixed solvent (3:2) silica gel column purification to obtain a slightly yellow solid tertiary alcohol (yield 88%) ; using DEI-MS to identify the compound, formula C 22 h 19 BrO, detection value [M+1] + = 379.03, calculated value 378.06;
[0063] Take...
Embodiment 2
[0064] Embodiment 2: the synthesis of intermediate A2:
[0065] synthetic route:
[0066]
[0067] Intermediate A2 was prepared according to the synthetic method of intermediate A1 in Example 1, the difference being that the compound bromobenzene was replaced by 1,1'-biphenyl;
[0068] Using DEI-MS to identify the compound, formula C 34 h 27 Br, detection value [M+1] + =514.85, calculated value 514.13.
Embodiment 3
[0069] Embodiment 3: the synthesis of intermediate A3:
[0070] synthetic route:
[0071]
[0072] Prepare intermediate A3 by the synthetic method of intermediate A1 among the embodiment 1, difference is to replace bromobenzene with benzene in the third step reaction;
[0073] Using DEI-MS to identify the compound, formula C 28 h 23 Br, detection value [M+1] + =438.10, calculated value 438.87.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com