Method for synthesis of aldehydes by hydroformylation of alkenes on one same set of production equipment
A technology for olefin hydroformylation to synthesize aldehydes and production equipment, which is applied in chemical instruments and methods, carbon monoxide reaction preparation, organic compound/hydride/coordination complex catalysts, etc., and can solve problems such as catalyst poisoning and reduced reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
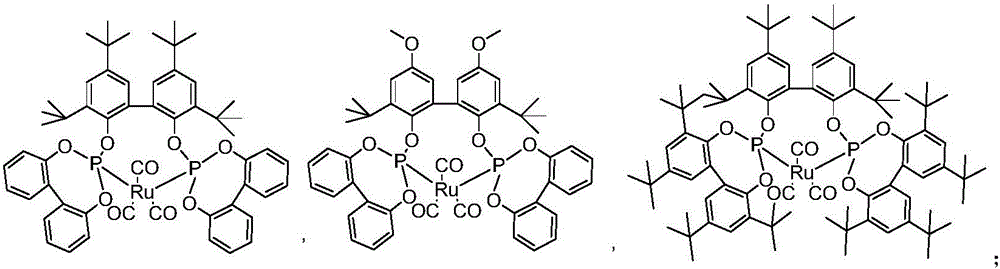
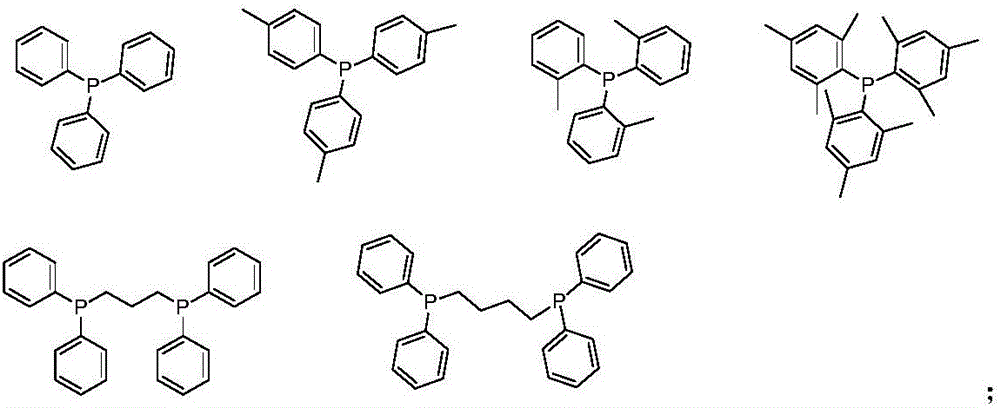
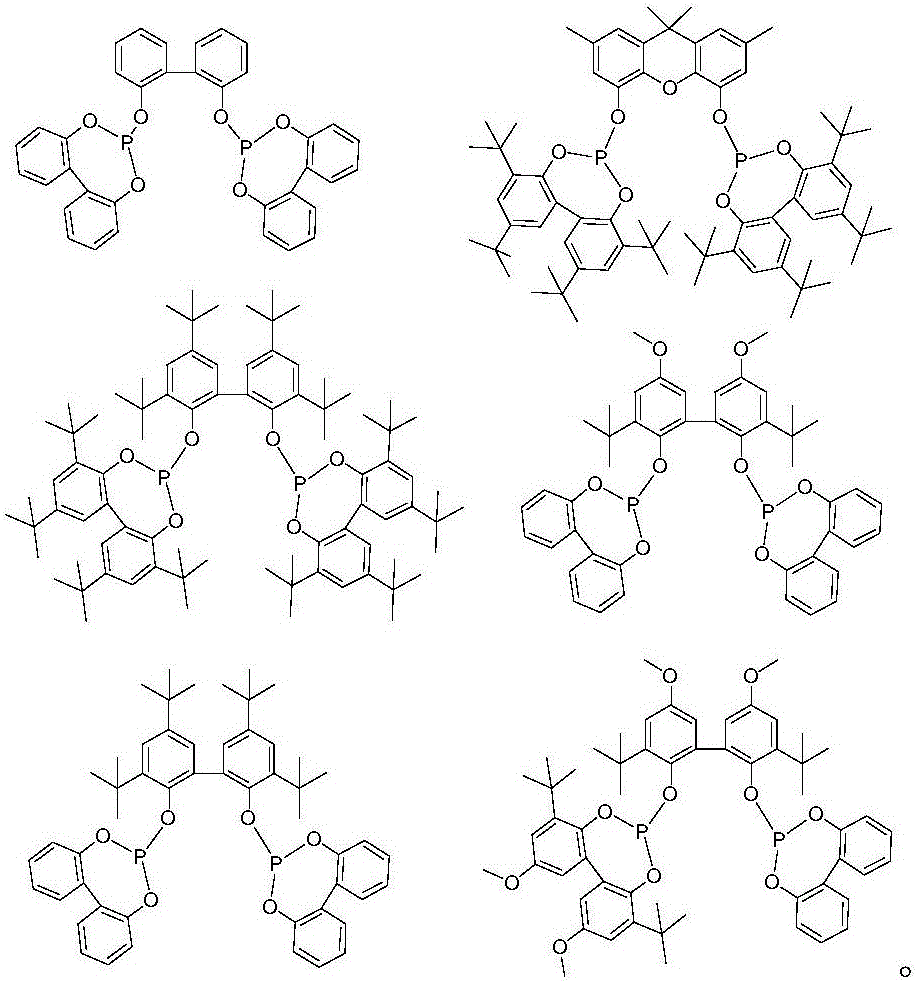
Image
Examples
Embodiment 1
[0025] Add 0.14 mmol [Rh(acac)(CO) to a 200 mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ], 0.56 mmol of L1 monophosphine ligand 0.56 mmol of L11 bidentate phosphite ligand, 0.07 mmol of Ru 3 (CO) 12 , and 70ml of isovaleraldehyde, feed 15g of isobutylene, connect the gas line, replace the gas in the kettle with synthesis gas (hydrogen: carbon monoxide=1:1 (molar ratio))) three times, stir with an electromagnetically driven mechanical stirrer, and heat Raise the temperature to 110°C inside the kettle, feed synthesis gas until the total pressure is 2.0MPa, react for 1.5h under the conditions of 110°C and 2.0MPa, and calculate the conversion rate of 93% based on isobutene. The selection of isovaleraldehyde produced by the reaction Sex is 98%.
Embodiment 2
[0027] Add 0.14 mmol [Rh(acac)(CO) to a 200 mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ], 0.70 mmol of L5 monophosphine ligand 0.56 mmol of L10 bidentate phosphite ligand, 0.07 mmol of Ru 3 (CO) 12 , and 70ml of isovaleraldehyde, feed 15g of isobutylene, connect the gas line, replace the gas in the kettle with synthesis gas (hydrogen: carbon monoxide=1:1 (molar ratio)) three times, stir with an electromagnetically driven mechanical stirrer, and heat up To the inner temperature of the kettle at 110°C, feed synthesis gas until the total pressure is 1.5MPa, react at 110°C and 1.5MPa for 1.5h, calculate the conversion rate of 90% based on isobutylene, and the selectivity of the isovaleraldehyde produced by the reaction 97%.
Embodiment 3
[0029] Add 0.14 mmol [Rh(acac)(CO) to a 200 mL stainless steel autoclave equipped with a pressure gauge under air atmosphere 2 ], 0.28 mmol of L6 monophosphine ligand 0.42 mmol of L11 bidentate phosphite ligand, 0.07 mmol of Ru 3 (CO) 12 , and 70ml of isovaleraldehyde, feed 15g of isobutylene, connect the gas line, replace the gas in the kettle with synthesis gas (hydrogen: carbon monoxide=1:1 (molar ratio)) three times, stir with an electromagnetically driven mechanical stirrer, and heat up To the internal temperature of the kettle at 110°C, feed synthesis gas until the total pressure is 2.0MPa, react at 110°C and 2.0MPa for 1.5h, the conversion rate is 95% based on isobutylene, the choice of isovaleraldehyde produced by the reaction Sex is 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com