A kind of method utilizing continuous flow reactor to produce 3-difluoromethoxy-5-fluorophenylboronic acid
A technology of difluoromethoxy and fluorobenzene boronic acid, which is applied in the synthesis field of producing 3-difluoromethoxy-5-fluorobenzene boronic acid, can solve the problem of low purity and yield of the target product, many side reactions, and high energy efficiency. problems such as large consumption, to achieve the effect of short reaction time, increase conversion rate, and reduce side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
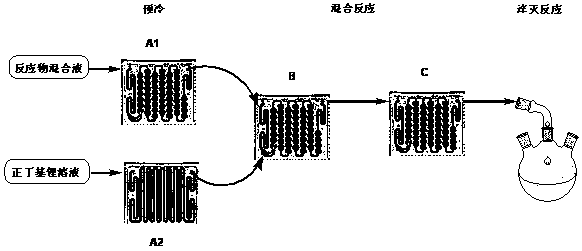
[0017] After the two microchannel reactors A1 and A2 are connected in parallel, they are connected in series with the two microchannel reactors B and C successively, and the microchannel reactors are replaced with anhydrous tetrahydrofuran;
[0018] Weigh 242 g of 3-difluoromethoxy-5-fluorobromobenzene and 226 g of triisopropyl borate and dissolve in 1500 g of tetrahydrofuran to form a homogeneous solution with a volume of about 1900 mL; weigh 1.6 M / L n-butyl Lithium n-hexane solution 810 mL;
[0019] Control the flow rate of the mixed solution of 3-difluoromethoxy-5-fluorobromobenzene to 80 mL / min; control the flow rate of n-butyllithium solution to 35 mL / min, and the two streams of materials flow through the A1 and A2 microreactions respectively The reactor is pre-cooled (-30°C), and then enters the microreactors B and C at the same time; the reaction residence time is 8.3s, and the reaction temperature is -30°C;
[0020] The outlet reaction solution was added dropwise to 1...
Embodiment 2
[0023] After the two microchannel reactors A1 and A2 are connected in parallel, they are connected in series with the two microchannel reactors B and C successively, and the microchannel reactors are replaced with anhydrous tetrahydrofuran;
[0024] Weigh 242 g of 3-difluoromethoxy-5-fluorobromobenzene and 125 g of trimethyl borate and dissolve in 1500 g of tetrahydrofuran to form a homogeneous solution with a volume of about 1900 mL; weigh 2.5 M / L n-butyllithium 600 mL of n-hexane solution;
[0025] Control the flow rate of the mixed solution of 3-difluoromethoxy-5-fluorobromobenzene to 80 mL / min; control the flow rate of n-butyllithium solution to 25 mL / min, and the two materials flow through the A1 and A2 microreactions respectively The reactor is pre-cooled (-30°C), and then enters the microreactors B and C at the same time; the reaction residence time is 9.1s, and the reaction temperature is -30°C;
[0026] The outlet reaction solution was added dropwise to 10% dilute hy...
Embodiment 3
[0028] After the two microchannel reactors A1 and A2 are connected in parallel, they are connected in series with the two microchannel reactors B and C successively, and the microchannel reactors are replaced with anhydrous tetrahydrofuran;
[0029] Weigh 242 g of 3-difluoromethoxy-5-fluorobromobenzene and 226 g of triisopropyl borate and dissolve in 1500 g of tetrahydrofuran to form a homogeneous solution with a volume of about 1900 mL; measure 2.5 M / L n-butyl Lithium n-hexane solution 600 mL;
[0030] Control the flow rate of the mixed solution of 3-difluoromethoxy-5-fluorobromobenzene to 80 mL / min; control the flow rate of n-butyllithium solution to 25 mL / min, and the two materials flow through the A1 and A2 microreactions respectively The reactor is pre-cooled (-10°C), and then enters the microreactors B and C at the same time; the reaction residence time is 9.1s, and the reaction temperature is -10°C;
[0031] The outlet reaction solution was added dropwise to 10% dilute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com