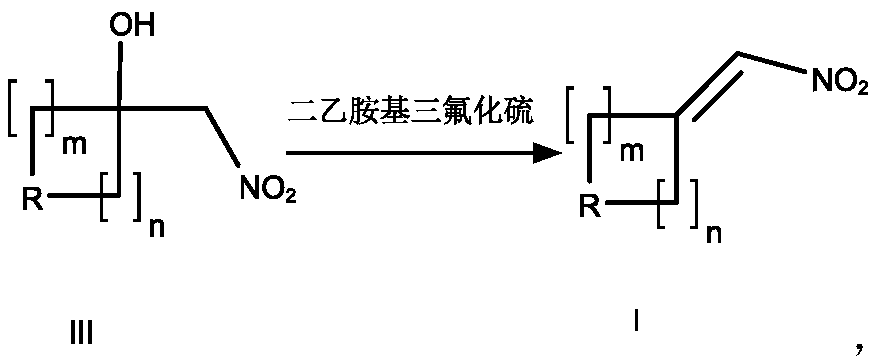
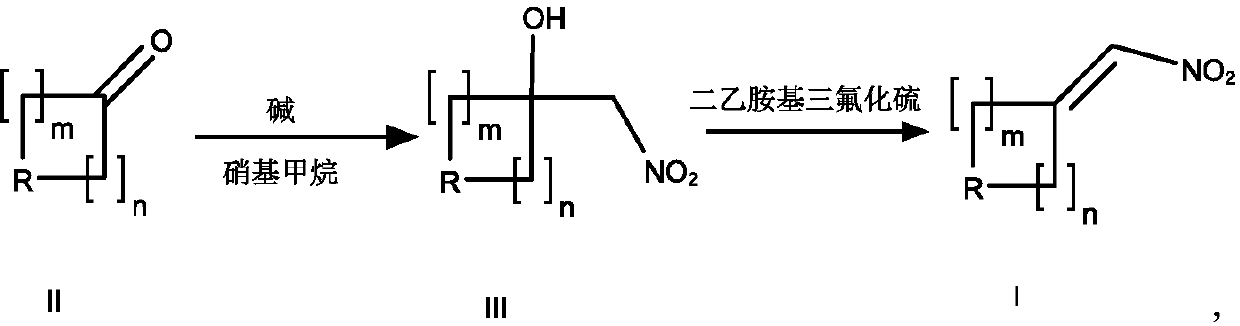
A method of using dast reagent as elimination reagent to synthesize conjugated nitroalkene substituted series derivatives
A compound and solvent technology, applied in the field of organic chemical synthesis, can solve problems such as potential safety hazards, unpleasant smells, and unfriendly environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
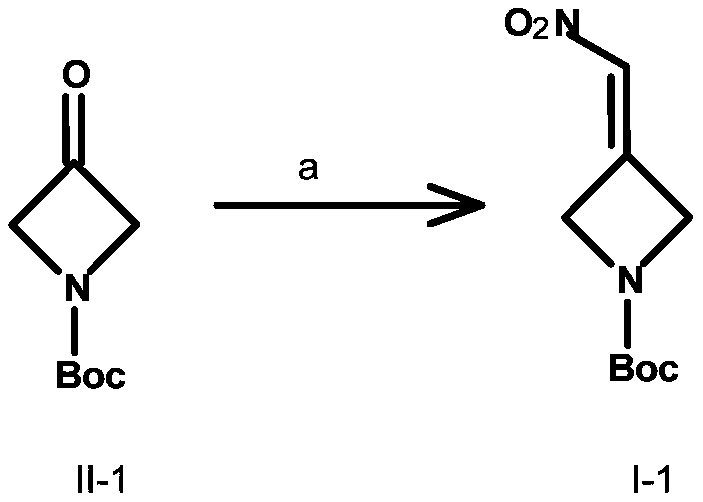
Embodiment 1
[0044]
[0045] Preparation of compound III-1:
[0046] Put compound II-1 (50.0g, 0.29mol, 1.0e.q.), 60mL nitromethane, 0.25mL DBU, and 300mL ethanol into a 500mL four-necked flask, and stir overnight at 25°C for 15h. TLC showed that there were remaining raw materials, and an additional 0.5 mLDBU, 20mL nitromethane, stirred for another 4 hours, TLC showed that the reaction was complete, concentrated to remove the solvent, added 200mL PE to the residue, put it in the refrigerator overnight, filtered, rinsed with PE, and the dried compound III-1 was a light yellow solid 62.04g, yield 92%.
[0047] Preparation of Compound I-1:
[0048] Compound III-1 (20.0g, 0.086mol, 1.0e.q.) was dissolved in 200mL DCM, DAST (20.8g, 0.129mol, 1.5e.q.) was added dropwise at 0°C, and the reaction was continued for 30min after the addition was complete. TLC detection showed that the raw materials reacted Completely, add 200mL water to quench the reaction, add saturated Na 2 CO 3 The aqueous ...
Embodiment 2
[0050]
[0051] Preparation of Compound III-2:
[0052] Compound II-2 (50.0g, 0.24mol, 1.0e.q.) was dissolved in 300mL of methanol, 80mL of nitromethane and sodium methoxide (0.65g) were added, and stirred overnight at room temperature for 15h. TLC showed that the reaction of the raw materials was complete. Concentrate to remove methanol , Pour the remaining viscous material into water, extract with EA, combine the organic phases, dry and concentrate, PE crystallize and filter with suction, and dry to obtain 58.45 g of compound III-2 as a yellow solid, with a yield of 90.1%.
[0053] Preparation of Compound I-2:
[0054] Compound III-2 (20.0 g, 0.075 mol, 1.0 e.q.) was dissolved in 200 mL of DCM, and DAST (20.8 g, 0.129 mol, 1.0 e.q.) was added dropwise at -78°C, and the stirring reaction was continued at 0°C for 1 h after the addition was completed. TLC detection showed that the reaction of the raw materials was complete, adding 200mL of saturated sodium bicarbonate to qu...
Embodiment 3
[0056]
[0057] Preparation of compound III-3:
[0058]Compound II-3 (50.0g, 0.22mol, 1.0e.q.) was dissolved in 300mL of methanol, 80mL of nitromethane and DIPEA (1.43g) were added, and stirred overnight at 25°C for 15h. TLC showed that the reaction of the raw materials was complete. After concentration and removal of methanol , Pour the remaining viscous material into water, extract with EA, combine the organic phases, dry and concentrate, PE crystallize and filter with suction, and dry to obtain 56.62 g of compound III-3 as a yellow solid, with a yield of 89.1%.
[0059] Preparation of Compound I-3:
[0060] Compound III-3 (20.0g, 0.0698mol, 1.0e.q.) was dissolved in 200mL 1,2-dichloroethane, DAST (13.51g, 0.0838mol, 1.2e.q.) was added dropwise at 0°C, and the addition was completed at 10°C Continue to stir the reaction for 1 h, TLC detection shows that the raw materials are completely reacted, the reaction is quenched by adding saturated potassium carbonate aqueous solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com