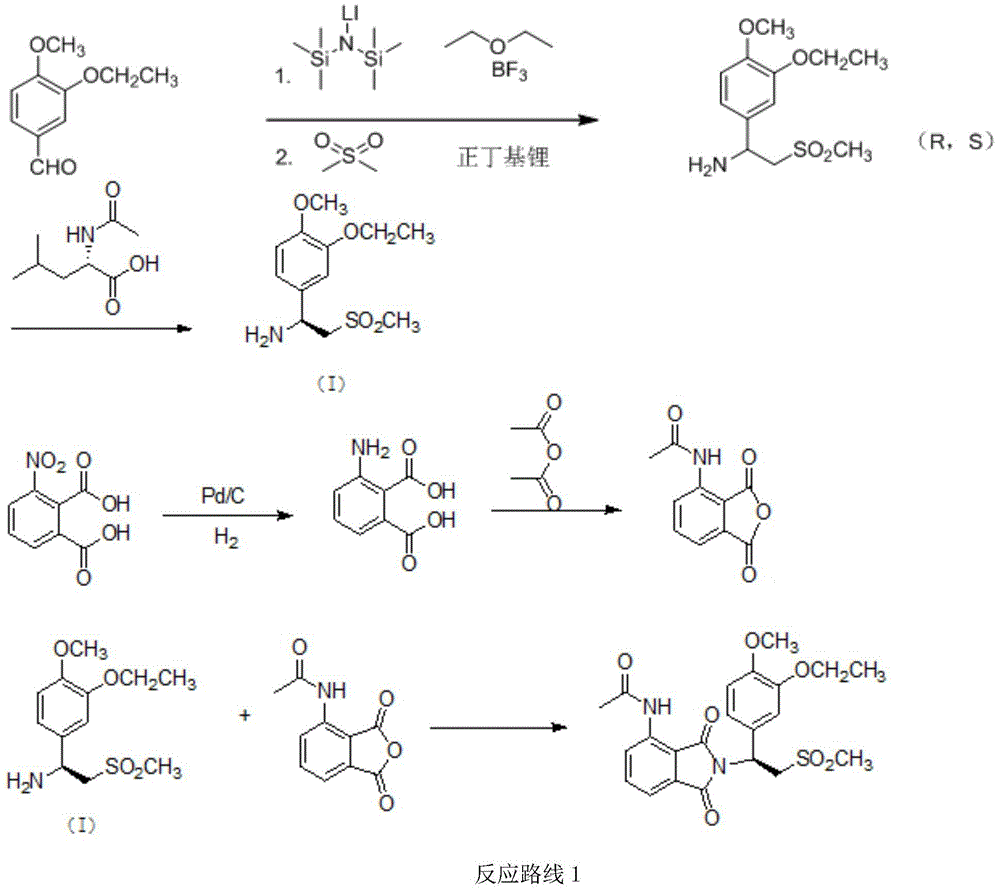
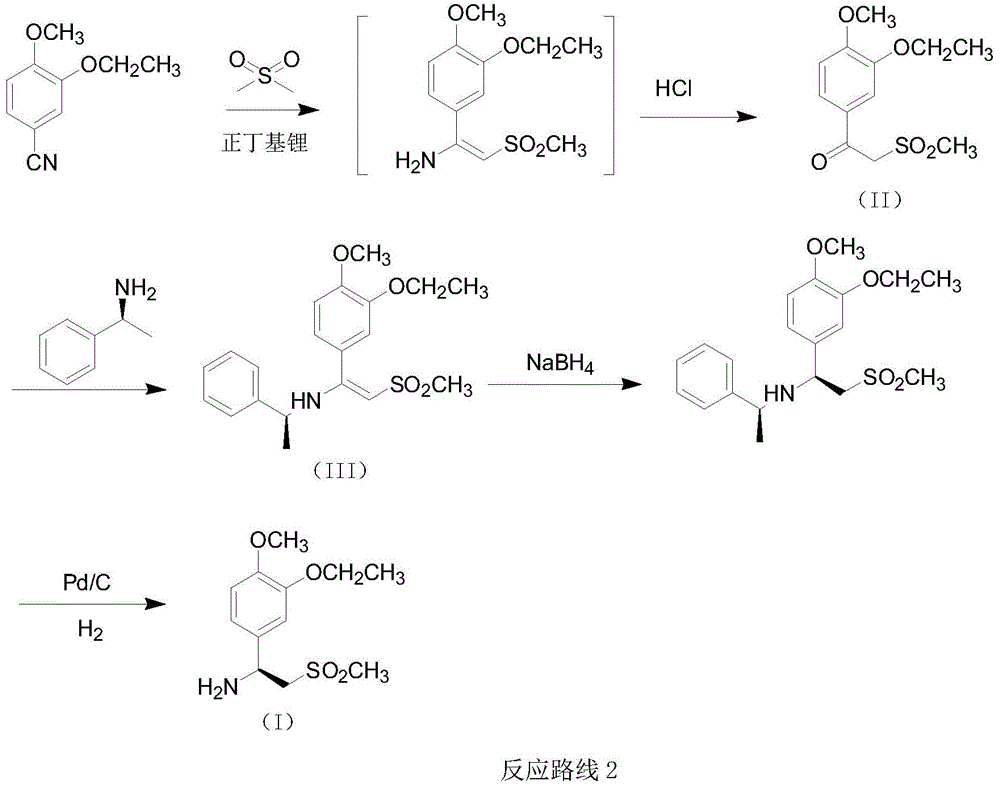
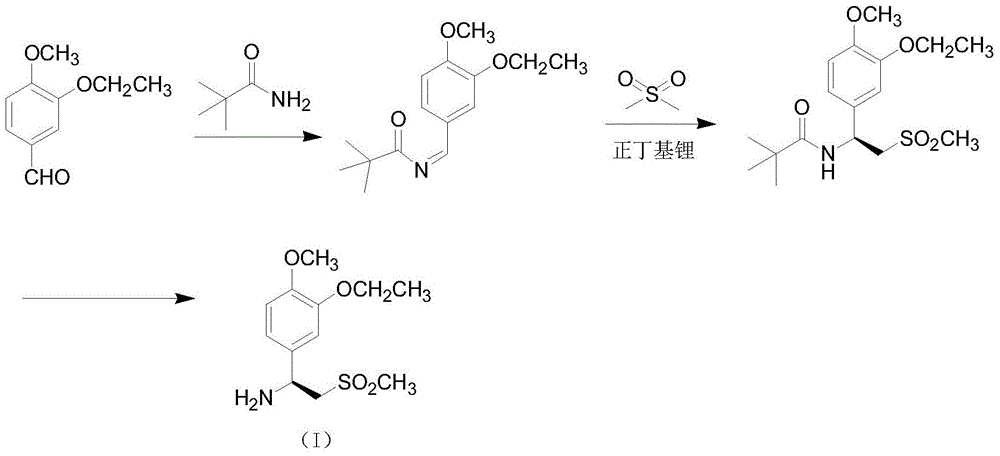
A kind of preparation method of synthetic Apremilast intermediate
A technology for intermediates and compounds, which is applied in the field of simple preparation of the synthetic apremilast intermediate-1-phenyl-2-methanesulfonylethylamine, which can solve the problem of reducing molecular utilization, difficult operation, and no industrial value, etc. problems, to achieve stable properties and low price, reduce waste water discharge, and facilitate operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Preparation of 1-(3-ethoxy-4-methoxy)phenyl-2-methanesulfonyl ethyl ketone (II)
[0065] Under the protection of nitrogen, add 200 grams of normal hexane and 24 grams (0.6 moles) of 60% solid sodium hydride successively in a dry 1000 milliliter glass flask, cool, keep the internal temperature between 15 ° C and 25 ° C, dropwise add 188 grams (2 mol) dimethyl sulfone, the dropwise addition is completed in about 2 hours, and then the temperature is raised to 35° C. for 1-2 hours (activation reaction). Then keep the internal temperature between 30°C and 35°C, add dropwise a mixed solution of 126.6 grams of 3-ethoxy-4-methoxybenzoic acid methyl ester (0.6 mole) and 200 grams of n-hexane, and drop it in about 2 hours. After dropping, react at 35°C for 4 hours. Cool down to 20°C, add 110 grams of saturated ammonium chloride aqueous solution dropwise, statically separate layers, extract the aqueous layer with 100 grams of ethyl acetate three times, combine the orga...
Embodiment 2
[0066] Example 2: Preparation of 1-(3-ethoxy-4-methoxy)phenyl-2-methanesulfonyl ethyl ketone (II)
[0067] Under the protection of nitrogen, add 200 g dimethyl sulfoxide, 200 g dimethyl sulfone, and 89.6 g (0.8 mole) potassium tert-butoxide to a dry 1000 ml glass flask in sequence, cool and keep the inner temperature between 10°C and 20°C After 2 hours, the temperature was raised to 30°C and the reaction was kept for 1 to 2 hours. Then keep the internal temperature between 33°C and 35°C, add 126.6 g of methyl 3-ethoxy-4-methoxybenzoate (0.6 mol) in batches, and react at 35°C for 4 hours after the addition. Cool down to 20°C, add 118 grams of saturated ammonium chloride aqueous solution dropwise, statically separate layers, extract the aqueous layer with 100 grams of ethyl acetate three times, combine the organic phases, dry with 30 grams of anhydrous sodium sulfate for 4 hours, filter, and recover the solvent from the filtrate 147.7 g of off-white solid 1-(3-ethoxy-4-methoxy)...
Embodiment 3
[0068] Example 3: Preparation of 1-(3-ethoxy-4-methoxy)phenyl-2-methanesulfonyl ethyl ketone (II)
[0069] Under the protection of nitrogen, add 180 grams of cyclohexane and 36 grams (0.9 moles) of 60% solid sodium hydride successively in a dry 1000 milliliter glass flask, cool, keep the internal temperature between 20°C and 25°C, and add dropwise 141 grams ( 1.5 moles) of dimethyl sulfone, the dropwise addition was completed in about 2 hours, and then the temperature was raised to 35°C and the reaction was kept for 1 to 2 hours. Then keep the internal temperature at 34-35°C, add dropwise a mixed solution of 134.4 grams of 3-ethoxy-4-methoxyethyl benzoate (0.6 moles) and 200 grams of cyclohexane, and drop it in about 2 hours. React at 35°C for 4 hours. Cool down to 20°C, add 165 grams of saturated ammonium chloride aqueous solution dropwise, statically separate layers, extract the aqueous layer with 150 grams of ethyl acetate three times, combine the organic phases, dry with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com