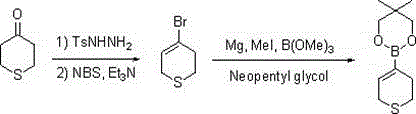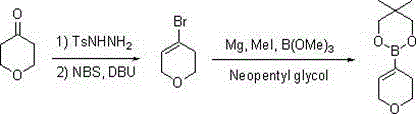A method for synthesizing 3,6-dihydro-2h-pyr(thia)pyran-4-boronate
A technology of boric acid ester and thiopyran, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high cost and affecting production efficiency, and achieve the effect of low cost, mild conditions and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
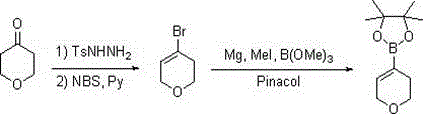
[0018] Synthesis of 3,6-dihydro-2H-pyran-4-boronic acid pinacol ester:
[0019]
[0020] Step 1: After mixing tetrahydropyran-4-one (10.0 g, 0.1 mol), p-toluenesulfonyl hydrazide (18.6 g, 0.1 mol) and 120 ml of ethanol, heat under reflux for reaction, and evaporate the solvent to dryness after the reaction is completed. Add pyridine (11.9 g, 0.15 mol) and 150 ml of dichloromethane directly, then add dropwise a solution of NBS (26.7 g, 0.15 mol) dissolved in 40 ml of dichloromethane, after the reaction is complete, add 10% hydrochloric acid to adjust the pH =2-3, separate the organic layer, evaporate the solvent to dryness under normal pressure, add 20 ml of sulfolane, and distill under reduced pressure to obtain 11.9 g of light yellow liquid: 3,6-dihydro-2H-pyran-4-bromo, GC: 98.2%, yield 73%;
[0021] Step 2: Add metal magnesium (1.9 g, 78 mmol) and 10 ml of tetrahydrofuran, add 2-3 drops of methyl iodide to trigger and start to drop 11.9 g of 3,6-dihydro-2H-pyran-4-bromo...
Embodiment 2
[0023] Synthesis of 3,6-dihydro-2H-pyran-4-boronic acid neopentyl glycol ester:
[0024]
[0025] Step 1: After mixing tetrahydropyran-4-one (10.0 g, 0.1 mol), p-toluenesulfonyl hydrazide (18.6 g, 0.1 mol) and 120 ml of ethanol, heat under reflux for reaction, and evaporate the solvent to dryness after the reaction is completed. Add DBU (22.8 g, 0.15 mol) and 150 ml of dichloromethane directly, then add dropwise a solution of NBS (26.7 g, 0.15 mol) dissolved in 40 ml of dichloromethane, after the reaction is complete, add 10% hydrochloric acid to adjust the pH =4-5, separate the organic layer, evaporate the solvent to dryness under normal pressure, add 20 ml of sulfolane, and distill under reduced pressure to obtain 12.2 g of light yellow liquid: 3,6-dihydro-2H-pyran-4-bromo, GC: 98.4%, yield 75%;
[0026] Step 2: Add metal magnesium (1.9 g, 78 mmol) and 10 ml of tetrahydrofuran, add 2-3 drops of methyl iodide to trigger and start to drop 12.2 g of 3,6-dihydro-2H-pyran-4-b...
Embodiment 3
[0028] Synthesis of 3,6-dihydro-2H-thiopyran-4-boronic acid pinacol ester:
[0029]
[0030] Step 1: After mixing tetrahydrothiopyran-4-one (10.0 g, 0.1 mol), p-toluenesulfonyl hydrazide (18.6 g, 0.1 mol) and 120 ml of ethanol, heat under reflux for reaction, and evaporate the solvent to dryness after the reaction is completed. Add pyridine (17.2 g, 0.22 mol) and 150 ml of dichloromethane directly, then add dropwise a solution of NBS (35.6 g, 0.20 mol) dissolved in 40 ml of dichloromethane, after the reaction is complete, add 10% hydrochloric acid to adjust the pH =2-3, separate the organic layer, evaporate the solvent to dryness under normal pressure, add 20 ml of sulfolane, and distill under reduced pressure to obtain 13.8 g of light yellow liquid: 3,6-dihydro-2H-thiopyran-4-bromo, GC: 98.7%, yield 77%;
[0031] Step 2: Add metal magnesium (2.2 g, 92 mmol) and 10 ml of tetrahydrofuran, add 2-3 drops of methyl iodide to trigger and start to drop 13.8 g of 3,6-dihydro-2H-p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com