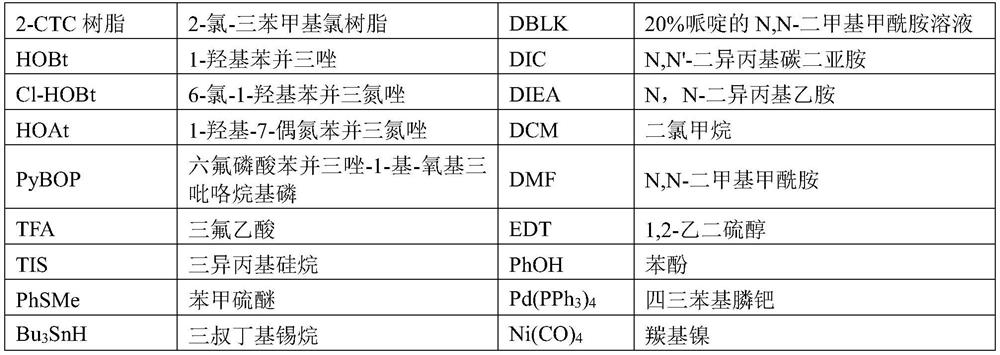
A kind of preparation method of glp-1 derivative
A synthesis method and amino acid technology, which can be used in peptide preparation methods, chemical instruments and methods, animal/human proteins, etc., can solve the problems of inability to guarantee the purity of fragmented peptides, cumbersome synthesis methods, and low purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047]The polypeptide fragment X is the 1st to the 4th amino acid, the polypeptide fragment Y is the 11th to the 16th amino acid, and the polypeptide fragment Z is the 24th to the 29th amino acid.
[0048] 1.1 Synthesis of polypeptide fragment X
[0049] Weigh 50g of 2-CTC resin with a degree of substitution of 1.0mmol / g, add it to the reactor of the solid-phase synthesizer, wash it twice with DMF, swell the resin with DMF for 30 minutes and then drain it, take 0.1mol Fmoc-Gly-OH for use Dissolve DMF, add to the above-mentioned reaction column with resin, add 0.4mol DIEA, drain after 6 hours of reaction, add 150ml of anhydrous methanol, seal for 1 hour, wash with DMF 6 times, and obtain Fmoc-Gly-2-CTC resin.
[0050] The Fmoc protecting group in the Fmoc-Gly-2-CTC resin was removed by DBLK twice for 15 minutes to obtain the H-Gly-2-CTC resin, which was then washed 6 times with DMF. Dissolve 0.15mol Fmoc-Glu(OtBu)-OH and 0.15mol HOBt in 150ml of DCM and DMF mixed solution wit...
Embodiment 2
[0082] The polypeptide fragment X is the 1st to the 4th amino acid, the polypeptide fragment Y is the 13th to the 16th amino acid, and the polypeptide fragment Z is the 26th to the 29th amino acid.
[0083] Using a synthesis method similar to that of Example 1, 53.4 g of liraglutide crude peptide was finally obtained, and the crude peptide yield was 88.1%. MALDI-TOF: (M+H)+=3752.1. Liraglutide refined peptide was obtained after purification: 19.8 g, purity: 98.7%, total yield: 32.7%, largest single impurity 0.12%.
Embodiment 3
[0085] The polypeptide fragment X is the 1st to the 4th amino acid, the polypeptide fragment Y is the 12th to the 16th amino acid, and the polypeptide fragment Z is the 25th to the 29th amino acid.
[0086] Using a synthesis method similar to Example 1, 53.9 g of liraglutide crude peptide was finally obtained, and the crude peptide yield was 88.1%. MALDI-TOF: (M+H)+=3752.1. Liraglutide refined peptide was obtained after purification: 20.2 g, purity: 99.0%, total yield: 33.4%, and the largest single impurity was 0.10%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com