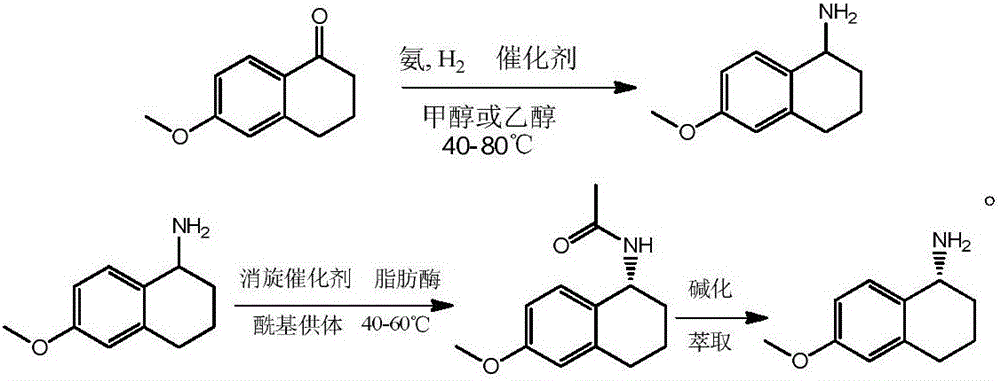
Synthesis and resolution of 6-methoxy-1-aminotetralin
A technology of methoxyl and tetralamine, applied in the field of synthesis and resolution of chiral amines, to achieve the effects of simple operation, high purity and good product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0007] 1) Preparation of 6-methoxy-1-tetralamine
[0008] In a 1000ml autoclave, add 88g of 6-methoxyl-1-tetralone, 600ml of absolute ethanol and 10g of catalyst SN-600P, seal the reaction kettle, use a vacuum pump to remove the air in the kettle, and then fill it with nitrogen to 0.5MPa, Then use a vacuum pump to evacuate; under negative pressure, charge 42.5g of ammonia gas, after the ammonia gas is filled, fill the autoclave with hydrogen to 3.5MPa, and heat up to 90°C for reaction. After reacting for 10 hours, it was found that the hydrogen pressure did not drop any more, so the reaction was stopped. After the temperature of the system dropped to room temperature, the reaction solution was filtered and concentrated to obtain crude 6-methoxy-1-tetralamine. Add the crude product to dilute hydrochloric acid solution while stirring, let it react to generate 6-methoxy-1-tetralamine hydrochloride, and dissolve it in the aqueous solution, and extract the aqueous solution with et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com