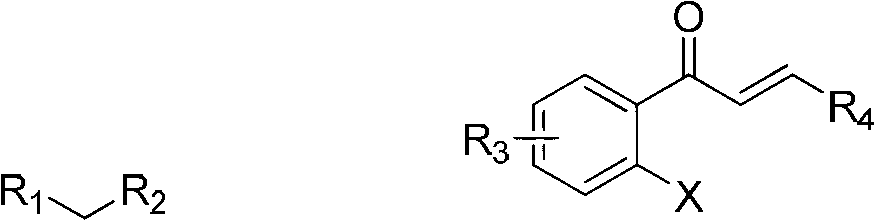Patents
Literature
41 results about "1-Tetralone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
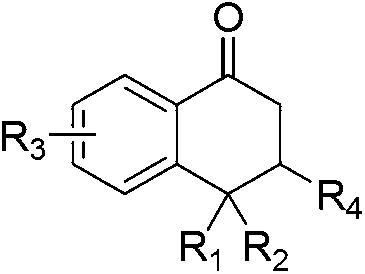
1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor. It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.
Polyamide-imide insulating varnish and preparation method thereof
The invention relates to a polyamide-imide insulating varnish. The polyamide-imide insulating varnish is characterized in that the varnish comprises the following components in parts by weight: 100-120 parts of polyamide-imide solution and 2-5 parts of sepiolite material, wherein the polyamide-imide solution mainly comprises the following components in parts by weight: 20-35 parts of polyamide-imide resin and 20-60 parts of organic solvent; the organic solvent is one or a combination of N-methylpyrrolidone, chlorobenzene, bromobenzene, dichlorobenzene, dibromobenzene, 1-tetralone, fenchone, phorone and isophorone; and the sepiolite material mainly comprises sepiolite. The polyamide-imide insulated enamel wire provided by the invention forms a homogeneous stable system, the surface friction coefficient is below 0.30, and each index meets the International Electrotechnical Commission (IEC) standard requirement; and the polyamide-imide insulating varnish is suitable to be used as the enamel wire insulating varnish for coating the surface of the copper wire.
Owner:GUANGDONG JINGDA REA SPECIAL ENAMELED WIRE CO LTD
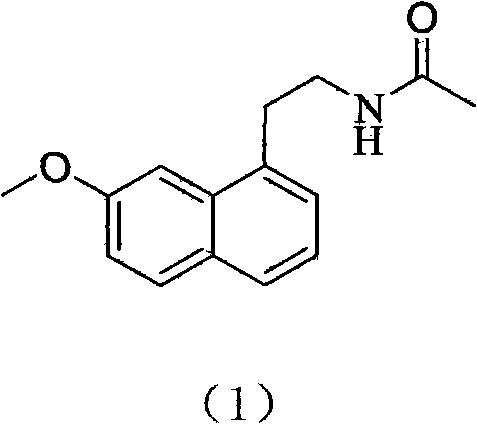
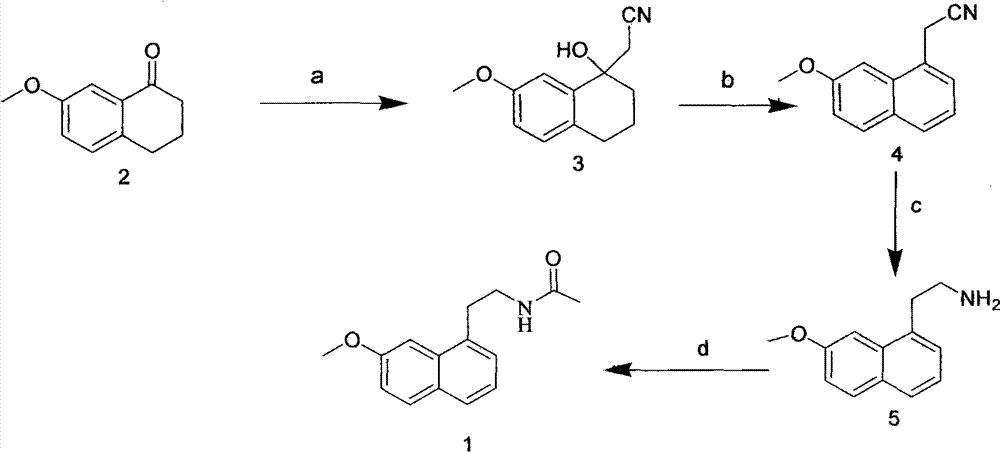
Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide
InactiveCN101759591AMeet the requirements of medicinal valueComply with purityOrganic active ingredientsNervous disorderDehydrogenationAntidepressants drugs
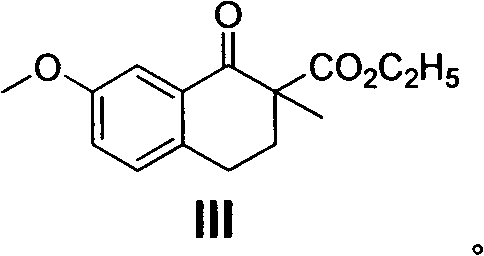
The present invention relates to a preparing method of agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) of melatonin antidepressant drugs of commercial production. Firstly, 7- anisyl-1-tetralone is used as raw material, and is prepared into 2-(1,2,3,4- tetrahydrochysene-1- oxhydryl-7- methoxynaphthalene-1-base) acetonitrile in a cyanophoric way; then, 2-(1,2- dihydro-1-oxhydryl-7- methoxynaphthalene-1-base) acetonitrile is generated in an aromatization dehydrogenation way, and (7-- anisyl-1- naphthyl) acetonitrile is generated in a backflow reaction dehydrogenation way; finally, the agomelatine (N-[2-(7- anisyl-1- naphthyl) ethide] acetamide) is generated by reducing and acetylizing in a one-kettle way. The present invention has the advantages of high product purity, friendly environment, convenient operation and low cost, and is suitable for the commercial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Preparation method of velpatasvir intermediate
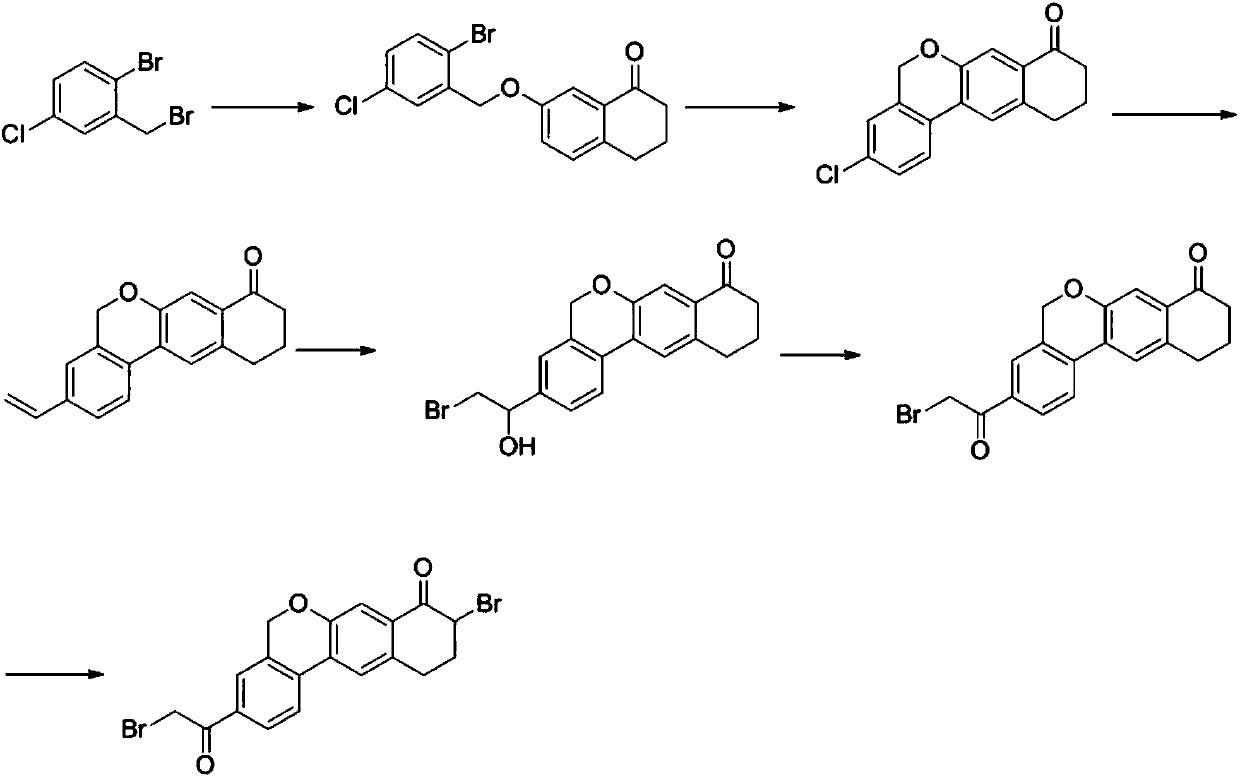
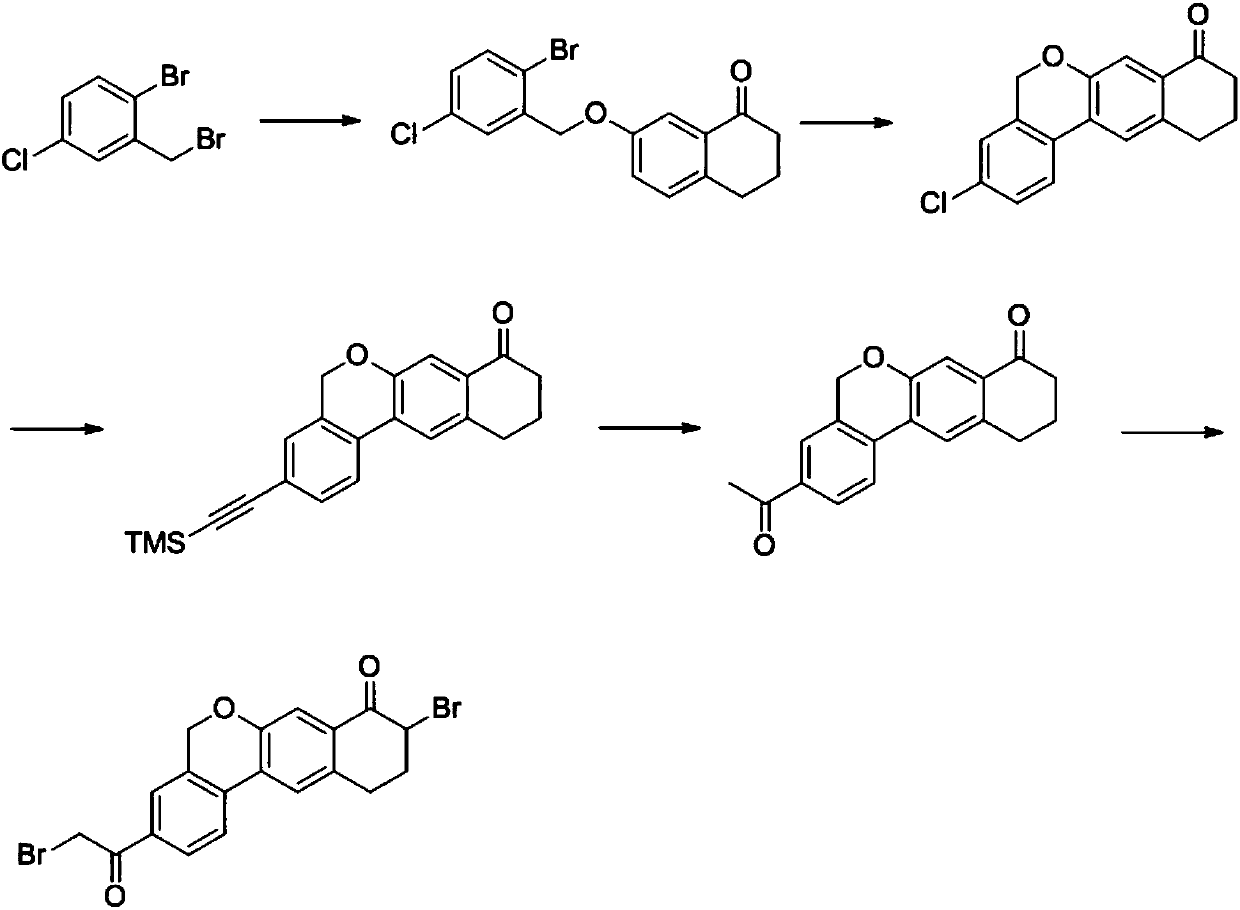
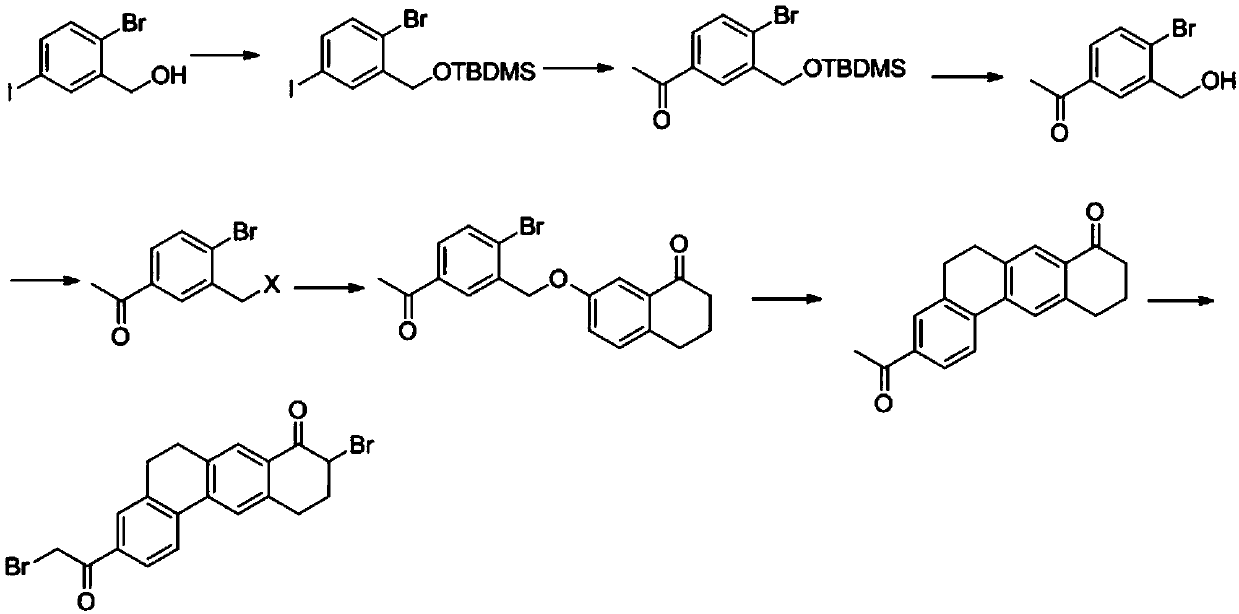
The invention provides a preparation method of a velpatasvir intermediate. According to the preparation method, ortho-toluidine is taken as an initial raw material, amino acetylation protection, Friedel-Crafts acylation, deamination protection, diazotization, and bromination are adopted so as to obtain 3-bromomethyl-4-bromoacetophenone; and alkylation with 7-hydroxy-1-tetralone, intramolecular coupling, and bromination are adopted so as to obtain 9-bromo-3-(2-bromoacetyl)-10, 11-dihydro-5H-benzo[d]naphtho[2,3-b]pyran-8(9H)-one. The yield and the purity of the velpatasvir intermediate preparedvia the preparation method are high, cost is low, and large size production is convenient to realize.
Owner:安徽省诚联医药科技有限公司
Polyamide imide insulating paint and preparation method thereof
Owner:GUANGDONG JINGDA REA SPECIAL ENAMELED WIRE CO LTD
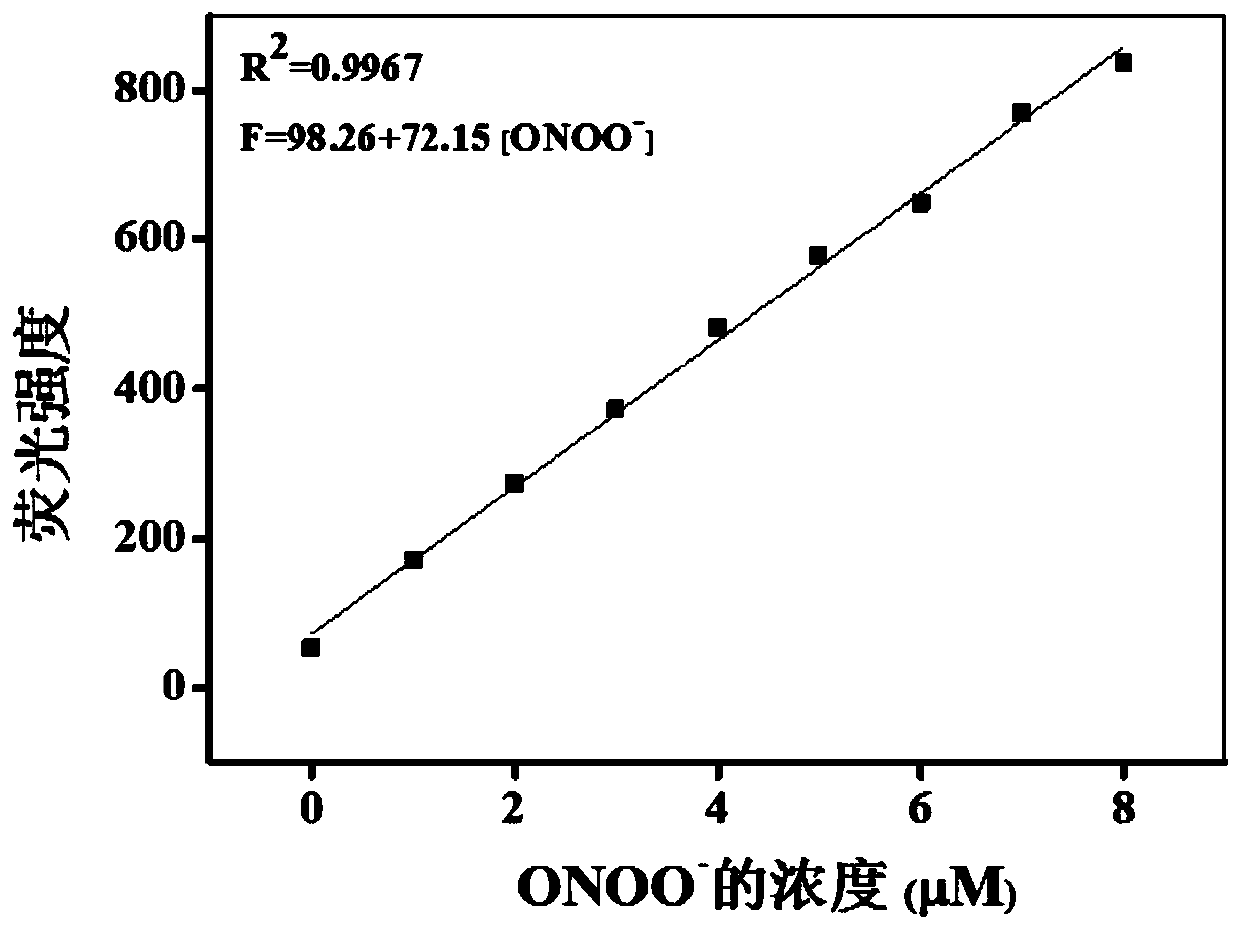
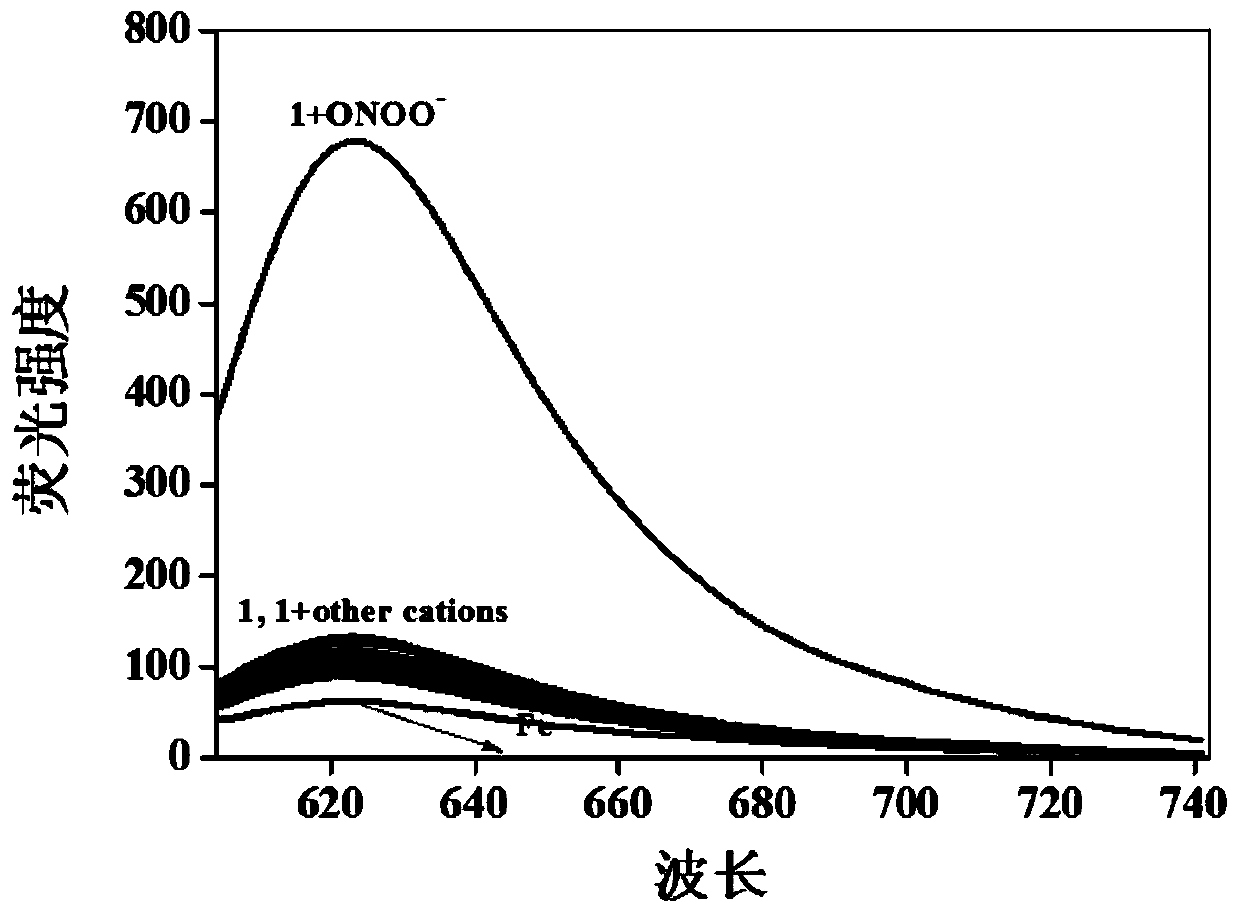
Near-infrared fluorescent probe for rapid detection of ONOO- and preparation method and application of near-infrared fluorescent probe for rapid detection of ONOO-
InactiveCN109897627AImprove solubilityGood dispersionGroup 3/13 element organic compoundsFluorescence/phosphorescenceDispersitySolubility
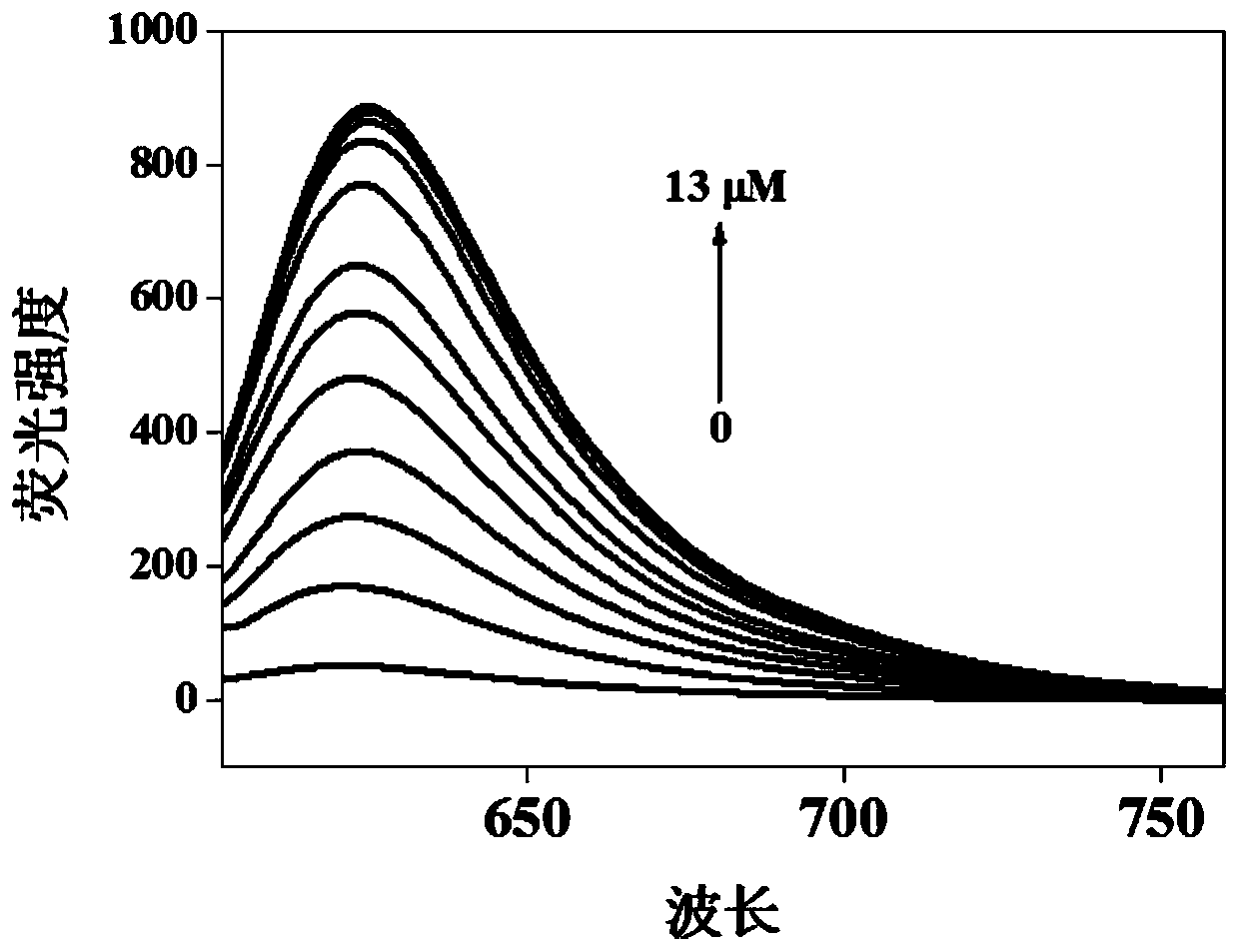
The invention provides a near-infrared fluorescent probe for rapid detection of peroxynitrite ions (ONOO-) and a preparation method and application of the near-infrared fluorescent probe for rapid detection of ONOO-. The preparation method includes steps: (1) weighing a certain mass of 6-hydroxy-1-tetralone and 4-(diethylamino)salicylaldehyde, dissolving in mixed solution of glacial acetic acid and concentrated sulfuric acid, ultrasonically well mixing, stirring overnight at the room temperature, quickly adding a product into ethyl acetate after reaction is completed, gradually separating outa red precipitate, filtering, washing with diethyl ether for three times, and performing vacuum drying to obtain a solid; (2) adding the obtained solid, potassium carbonate and 4-(bromomethyl)benzeneboronic acid pinacol ester into DMF solution, performing reaction at 60-80 DEG C for 5 hours, adding mixed solution into ice water after reaction is finished, extracting with chloroform, performing spin drying through a rotary evaporator, and carrying out column chromatography separation and purification to obtain a target product. The obtained probe is high in solubility and dispersity in aqueoussolution and can be used for rapid detection of ONOO- and cell imaging.
Owner:SHANXI UNIV
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS20060052639A1Easy to handleOrganic compound preparationCarboxylic acid amides preparationTetraloneHydrogen
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
Method for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide in tail gas of oil burning boiler
InactiveCN105148713AStrong complexing abilityFast precipitationDispersed particle separationCyclohexanoneTert-Butyloxycarbonyl protecting group
The invention relates to a method for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide in tail gas of an oil burning boiler. The method comprises the steps of introducing a sufficient amount of desulfurizing agent into the tail gas of the oil burning boiler through a pipeline, carrying out reaction on sulfur dioxide, sulfur trioxide, hydrogen sulfide and the desulfurizing agent to obtain complex precipitates, and filtering for removing the complex precipitates. The desulfurizing agent comprises the following components by weight percent: 2-ethoxybenzaldehyde, 4-methoxybenzyl bromide, cyclohexanone, triethylamine, 3-buten-1-ol, 1-tetralone, tritolyl phosphate, 3-dimethylaminopropylamine, 2-methoxy-5-fluorouracil, isopropylamine, 1-boc-3-piperidone, ethyl oxalyl monochloride and ethyl alcohol absolute. According to the method, the complexing capability with target substances is strong, the complex precipitate formation speed is high, the removal rate can up to 99%, the cost is low, the using amount is small, and scaling cannot occur, so that the environment cannot be damaged.
Owner:叶澄
Preparation method of 7-hydroxy-1-tetralone
ActiveCN106977377AReduce usageImprove friendlinessOrganic compound preparationPreparation from carboxylic acid anhydridesStructural formulaButyric acid
The invention relates to a preparation method of 7-hydroxy-1-tetralone with a structural formula I. 4-(4-methoxyphenyl)butyric acid with a structural formula II is used as a starting material, in the presence of Lewis acid, a one pot process is used for carrying out cyclization and a demethylating reaction, and 7-hydroxy-1-tetralone is obtained. The preparation method has simple operation, phosphorus-containing reagents are not used, and environmental friendliness of the reaction is improved; in addition, yield of 7-hydroxy-1-tetralone is high (higher than 85%), and purity is good (high than 99.9%). Accordingly the method is completely suitable for industrial production, and satisfies requirements on quality of intermediates in medical industry.
Owner:JIANGXI SYNERGY PHARMA
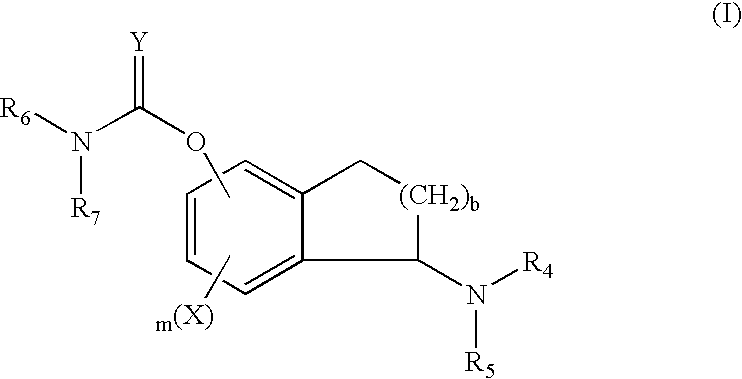
Pyrrolidinyl hydroxamic acid compounds and their production process
A compound of the formula: and it pharmaceutically acceptable salt, wherein A is hydrogen, hydroxy or OY, where Y is a hydroxy protecting group; Ar is phenyl optionally substituted with one or more substituents selected from halo, hydroxy, C1-C4 alkyl, C1-C4 alkoxy, CF3, C1-C4 alkyloxy, and carboxy-C1-C4 alkyloxy; X is phenyl, naphthyl, biphenyl, indanyl, benzofuranyl, benzothiopheny, 1-tetralone-6-yl, C1-C4 alkylenedioxy, pyridyl, furyl and thienyl, these groups optionally being substituted with up to three substituents selected from halo, C1-C4 alkyl, C1-C4 alkoxy, hydroxy, NO2, CF3 and SO2CH3; and R is hydrogen, C1-C4 alkyl or a hydroxy protecting group. These compounds and pharmaceutical compositions containing them are useful as analgesic, antiinflammatory, diuretic, anesthetic or neuroprotective agents, or an agent for stroke or treatment of functional bowel diseases such as abdominal pain, for the treatment of a mammalian subject, especially a human subject. Further, the present invention provides processes for producing the hydroxamic compounds of formula (I) and their intermediate compounds of formula (II).
Owner:PFIZER INC
Treating agent for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide from tail gas of oil burning boiler
InactiveCN105148694AStrong complexing abilityFast precipitationDispersed particle separationCyclohexanoneBenzoyl bromide
The invention relates to a treating agent for removing sulfur dioxide, sulfur trioxide and hydrogen sulfide from tail gas of an oil burning boiler. The treating agent is prepared by compounding following components: 2-ethoxy benzaldehyde, 4-methoxy benzyl bromide, cyclohexanone, N,N-diethylethanamine, 3-butene-1-alcohol, 1-tetralone, tricresyl phosphate, N,N-dimethyl trimethylene diamine, 2-methoxy-5-fluorouracil, isopropamide, N-butyloxycarboryl-3-piperidone, ethyl chlorooxoacetate, N,N-dimethyl methylamine, 2-bromine-4'-methylacetophenone, quinoline-2-formaldehyde, absolute ethyl alcohol, and the like. The treating agent is strong in complexing power with target matters, the speed of forming complex and sediment is high, the removal rate can reach 99%, the cost is low, the dosage is less, no scaling is formed and no damage is caused to the environment.
Owner:叶澄
Nanosphere-metal composite catalyst, preparation method thereof, application and preparation method of 5-hydroxyl-1-tetralone
ActiveCN108722396ARegular and orderlyLarge specific surface areaPreparation by hydroxy compound hydrogenationOrganic compound preparationMicrosphereHydrogenation reaction
The invention provides a nanosphere-metal composite catalyst. The nanosphere-metal composite catalyst is prepared from SiO2 nanospheres and Pd metal particles loaded on the surfaces of the SiO2 nanospheres. The nanosphere-metal composite catalyst provided by the invention has higher catalytic activity and selectivity and can catalyze hydrogenation reaction by high efficiency. The invention also provides a preparation method of the catalyst; the catalyst can be prepared by an impregnation method, the preparation method is simple, the operation is easy and the cost is low; the nanosphere-metal composite catalyst provided by the invention is utilized to catalyze hydrogenation reaction of 1,5-dihydroxynaphthalene, the reaction selectivity is high and the yield of 5-hydroxyl-1-tetralone is high.
Owner:HANGZHOU LUPU BIOTECH CO LTD
Method for synthesizing saprorthoquinone
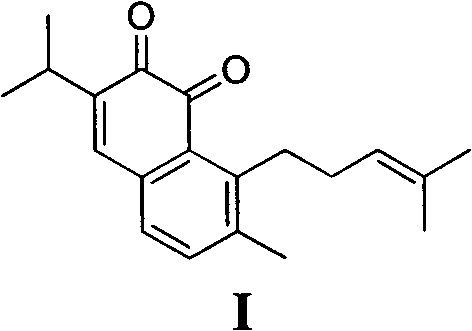
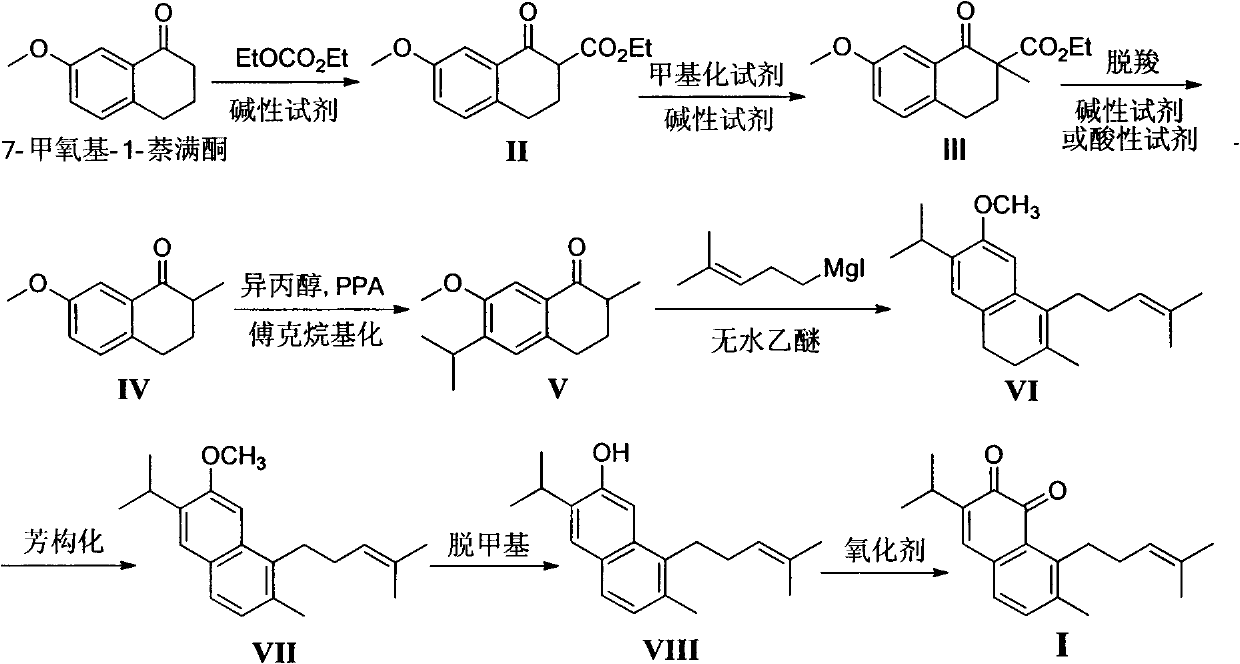
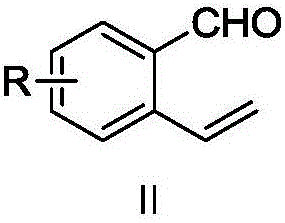
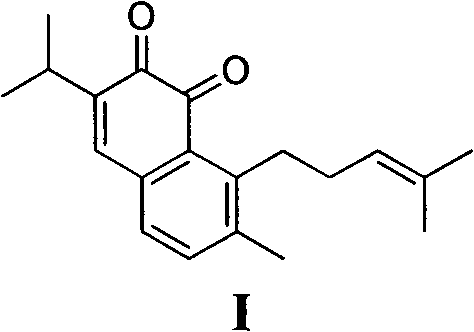
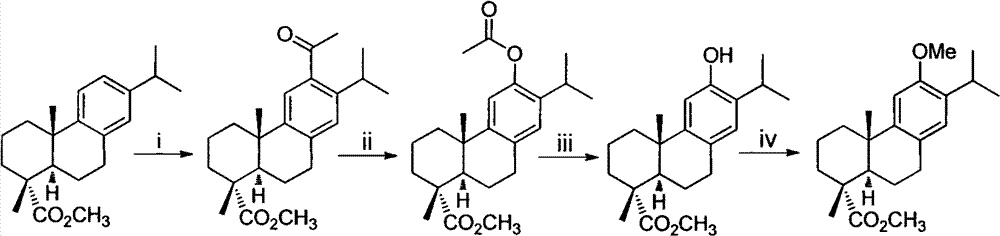
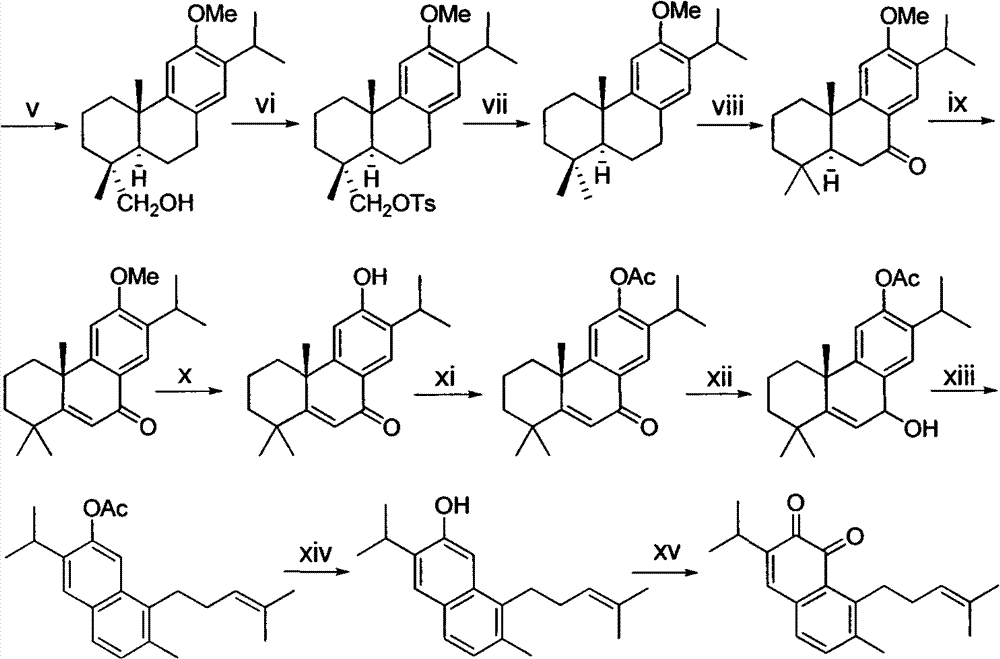
The invention belongs to the field of chemical synthesis, and in particular relates to a method for synthesizing saprorthoquinone which is shown as a formula (I) and serves as a natural product with high antitumor activity. The method comprises the following steps of: performing carbonyl alpha-site methylation, selective isopropyl substitution of an aromatic ring and Grignard reaction on 7-methoxyl-1-tetralone serving as an initiative raw material to prepare a key intermediate, namely 1-(4-methyl-3-pentenyl)-2-methyl-6-isopropyl-7-methoxyl-3,4-dihydronaphthalene; and aromatizing with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), demethylating with ethanethiol and oxidizing with 2-iodoxybenzoic acid (IBX) to prepare the saprorthoquinone.
Owner:CHINA PHARM UNIV
Preparation process of montelukast sodium and its intermediate product
ActiveCN104293850BEfficient reuseReduce typesOrganic chemistryChemical recyclingMethylmagnesium chlorideCoupling
The present invention discloses a montelukast sodium preparation technology and intermediates; 7-chloro-2-methylquinine and 3-bromobenzaldehyde are used as raw materials for condensation reaction to obtain a compound A2; by carbon-carbon coupling of the compound A2 and 1-tetralone in the presence of a catalyst, an intermediate compound A3 is obtained; an important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric Baeyer-villiger reaction, a chiral center is highly selectively constructed, an important intermediate compound A5 is prepared from the compound A4 by grignard reaction by use of methylmagnesium chloride, finally montelukast sodium (A6) is obtained; according to the technology, the highly chiral important intermediate compound A4 is obtained by bio-enzyme catalyzed asymmetric reaction, the catalyst can be effectively repeatedly used, and the kind of used solvents is less, and the montelukast sodium preparation technology has the characteristics of safety and environmental protection, greatly saves the production cycle, is low in production cost, high in total yield, and simple in operation of production units, and is suitable for industrialized production.
Owner:JIANGSU HANSYN PHARMA
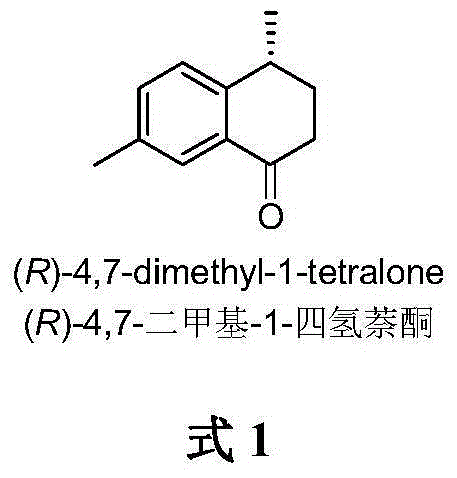
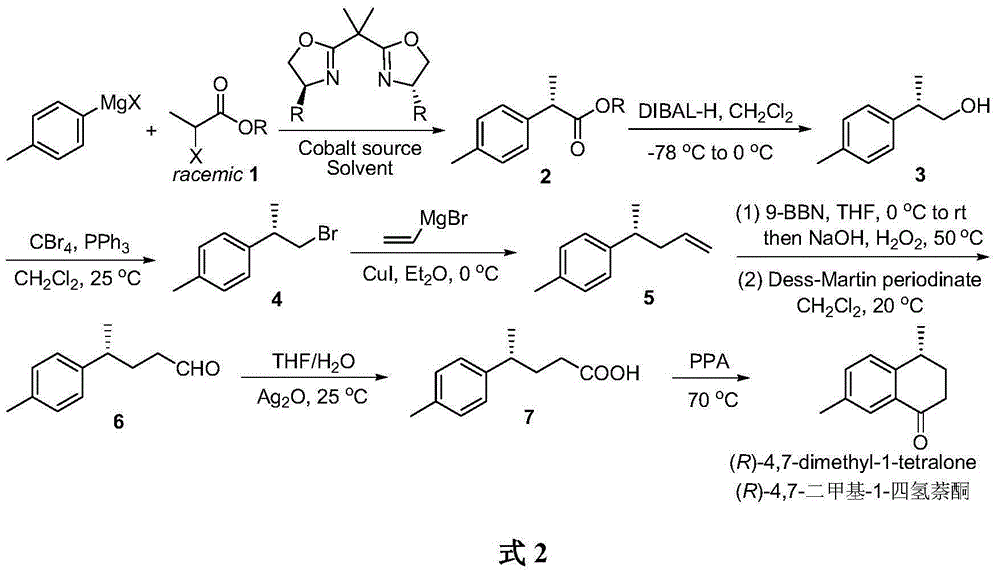
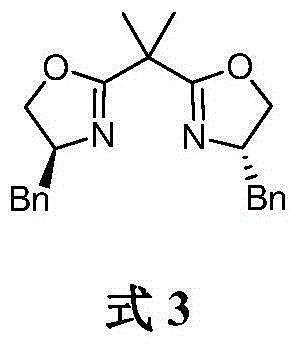
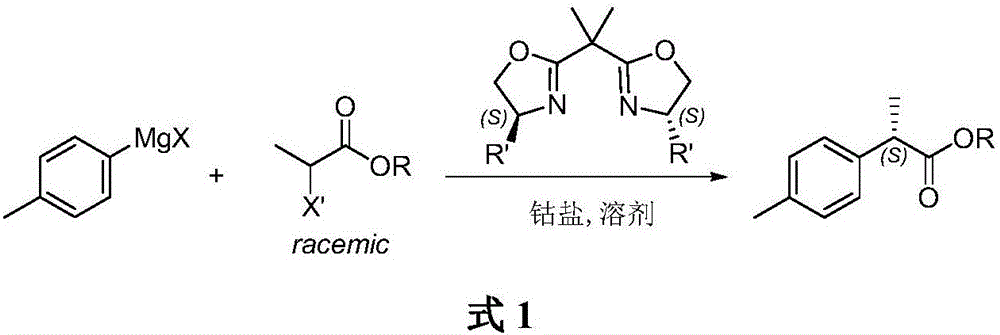
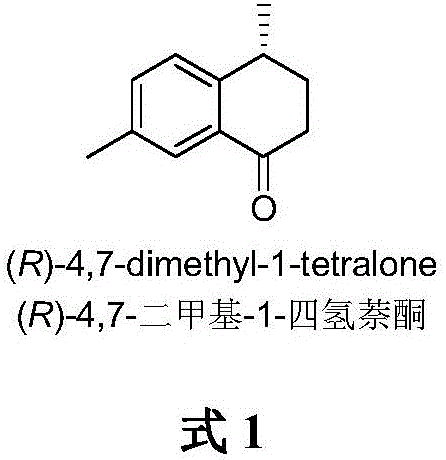
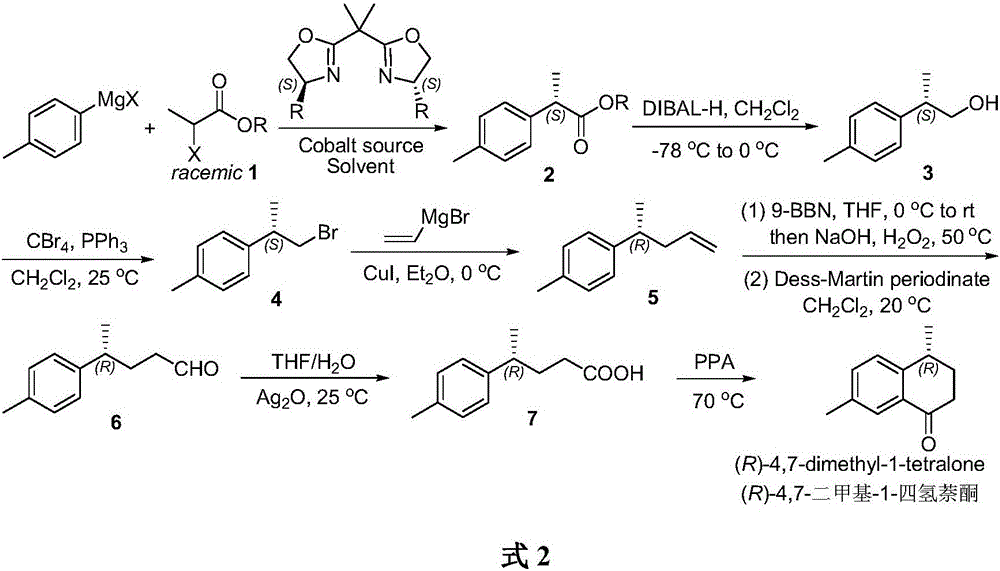
Method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone
The invention discloses a method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone. According to the method, an asymmetrical Kumada cross coupling reaction is conducted on racemization 2-halogenated propionate ester catalyzed by bis oxazoline / cobalt and a methyl phenyl grignard reagent, and (S)-P-toluene propionate ester 2 is firstly generated; then, (S)-P-toluene propionate ester 2 is reduced to (S)-P-toluene propyl alcohol 3 through diisobutylaluminium hydride (DIBAL-H), and then (R)-4-p-methylphenyl-1-amylene 5 is obtained through bromine generation and coupling with and vinyl grignard reagent; next, a hydroboration-oxidation reaction and a Dess-Martin oxidizing reaction are sequentially conducted, and (R)-4-p-methylphenyl valeraldehyde 6 is obtained; finally, oxidation is conducted through silver oxide, an intramolecular Fourier acyl reaction is conducted, and (R)-4,7-dimethyl-1-tetralone is obtained in a ring-closure synthesis mode. The synthesis route is simple and concise, 8 reactions are conducted in all, the total yield is 27%, and the optical purity of a product is 90%.
Owner:CHINA AGRI UNIV
Synthetic method for agomelatine
InactiveCN101792400BLow costReduce consumptionOrganic compound preparationCarboxylic acid amides preparationTetralinALUMINUM HYDRIDE
The invention relates to a synthetic method for agomelatine. The method comprises the following steps: reacting 7-methoxy-1-tetralone (2) serving as a raw material with acetonitrile under the action of n-butyl lithium to obtain 1-hydroxy-7-methoxy-1,2,3,4-tetralin-1-naphthyl acetonitrile (3); then uniformly mixing the compound (3) and acetic acid solvent or toluene, adding dichloro dicyano benzoquinone into the mixture for reacting at the temperature of between 50 and 150 DEG C for 4 to 20 hours to obtain a compound (4); adding the compound (4) into uniformly mixed solution of lithium aluminum hydride and tetrahydrofuran for reacting at the temperature of between 0 and 60 DEG C for 5 to 24 hours to obtain (7-methoxy-1-naphthyl) ethylamine (5); and finally uniformly mixing the compound (5) and triethylamine or naphthyridine, and adding acetylchloride into the mixture for reacting at the temperature of between 0 and 25 DEG C for 1 to 5 hours to obtain the agomelatine (1). The synthetic method for the agomelatine of the invention has the advantages of high yield, low cost, high controllability, simple processing after the reaction and environment protection and is suitable for industrial production of the melatonin antidepressant.
Owner:EAST CHINA NORMAL UNIV
Chlorine-resistant fiber cloth for swimsuits
The invention discloses long-acting chlorine-resistant fiber cloth for swimsuits. The chlorine-resistant fiber cloth comprises, by weight, 115 parts of polyester fibers, 15 parts of nanometer titanium dioxide, 1 part of tetrakis hydroxymethyl phosphonium sulfate, 2 parts of 5- hydroxy -1-tetralone, 2 parts of 4-cyclohexanone carboxylate, 1.5 parts of 2',4'-acetylphosphoramidothioate acetanilide, 3 parts of sodium pentanesulfonate, 2 parts of 4-isopropyl benzene sulfonyl chloride, 5 parts of 5-amino-2-methyl benzene sulfonic acid, 5 parts of sulfosuccinate butanedioic acid dioctyl phthalate sodium salt, 1 part of softener and 1 part of antioxidants.
Owner:JINJIANG HONGXING FASHION WEAVING
Process for producing 1,5-diaminonaphthalene
A process for producing 1,5-diaminonaphthalene without formation of 1,8-diaminonaphthalene and not through an unstable nitro imine and nitro enamine as intermediates, the process including the steps of dehydrogenating 5-substituted-1-tetralone to produce a naphtol compound and then aminating the hydroxyl group of the naphtol compound.
Owner:MITSUI CHEMICALS POLYURETHANES INC
Method for refining 4 ¿C (3,4 ¿C dichlorobenzene group) ¿C 1 ¿C tetralin ketone
ActiveCN101050169ALow costReduce production processing timeCarbonyl compound separation/purificationTetralinKetone
This invention discloses a method for refining 4-(3,4-dichlorophenyl)-1-tetralone. The method comprises: adding 4-(3,4-dichlorophenyl)-1-tetralone and solvent at a ratio of 1:(3-4) into a three-necked flask with a stirrer, heating under stirring until 4-(3,4-dichlorophenyl)-1-tetralone is completely dissolved, heating to 60-100 deg.C, refluxing for 0.5-2 h, cooling the filtrate to -5-10 deg.C, filtering, recrystallizing to obtain refined 4-(3,4-dichlorophenyl)-1-tetralone, and drying at 40-80 deg.C to obtain dry 4-(3,4-dichlorophenyl)-1-tetralone. The method has such advantages as high purity, short time, high yield, and stable product quality.
Owner:DAQING PETROLEUM ADMINISTRATION +1
Synthesis method of levo-amino compound
InactiveCN106467476AEasy to operateRaw materials are easy to getOrganic compound preparationOrganic chemistry methodsHydroxylamineSynthesis methods
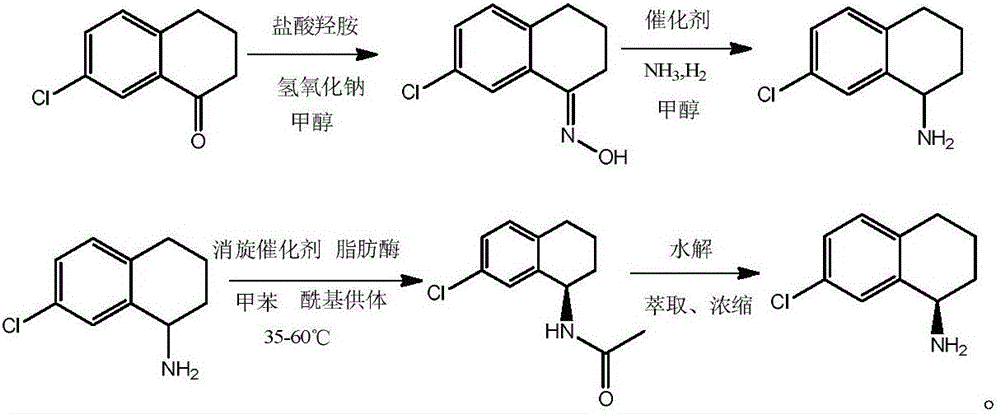
The invention discloses a synthesis method of a levo-amino compound S-7-chloro-1,2,3,4-tetrahydronaphthalene-1-amine. The method includes the steps of: performing a reaction to a raw material, 7-chloro-1-tetralone, with hydroxylamine to generate oxime; performing hydrogenation reduction to the oxime to prepare racemized amine; performing a one-pot dynamic kinetic resolution reaction to the product amine with lipase, a racemization catalyst and an acyl group donor; and hydrolyzing a resolution reaction product to prepare the S-7-chloro-1,2,3,4-tetrahydronaphthalene-1-amine. The method has simple operations, high product yield, high optical purity of the resolution product, and the like.
Owner:王际菊
Method for supercritical separation and purification of 4,8-dht from pecan exocarp
ActiveCN104292093BInhibition of germinationGrowth inhibitionPlant growth regulatorsCarbonyl compound separation/purificationPlanting seedSolvent
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Chlorine-resistant fiber cloth for swimwear
The invention discloses long-acting chlorine-resistant fiber cloth for swimsuits. The chlorine-resistant fiber cloth comprises, by weight, 115 parts of polyester fibers, 15 parts of nanometer titanium dioxide, 1 part of tetrakis hydroxymethyl phosphonium sulfate, 2 parts of 5- hydroxy -1-tetralone, 2 parts of 4-cyclohexanone carboxylate, 1.5 parts of 2',4'-acetylphosphoramidothioate acetanilide, 3 parts of sodium pentanesulfonate, 2 parts of 4-isopropyl benzene sulfonyl chloride, 5 parts of 5-amino-2-methyl benzene sulfonic acid, 5 parts of sulfosuccinate butanedioic acid dioctyl phthalate sodium salt, 1 part of softener and 1 part of antioxidants.
Owner:JINJIANG HONGXING FASHION WEAVING
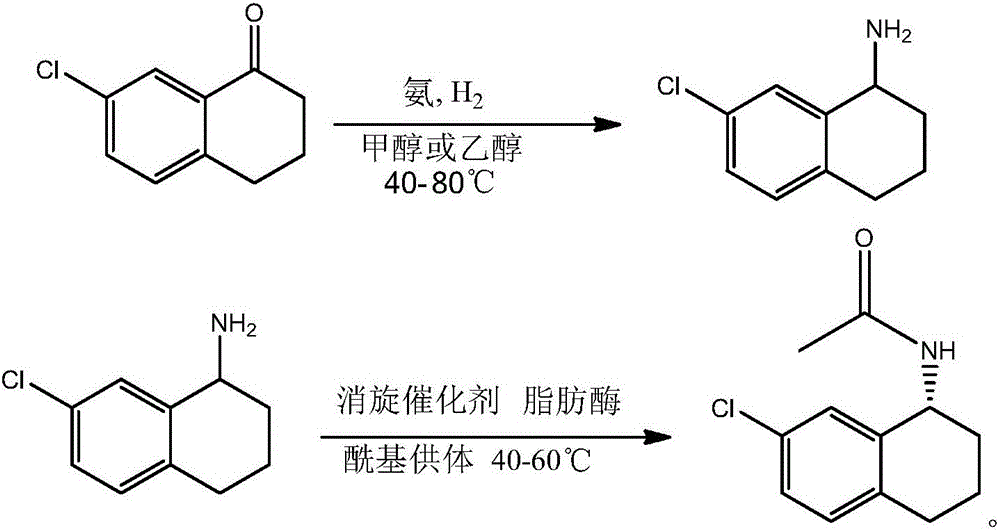
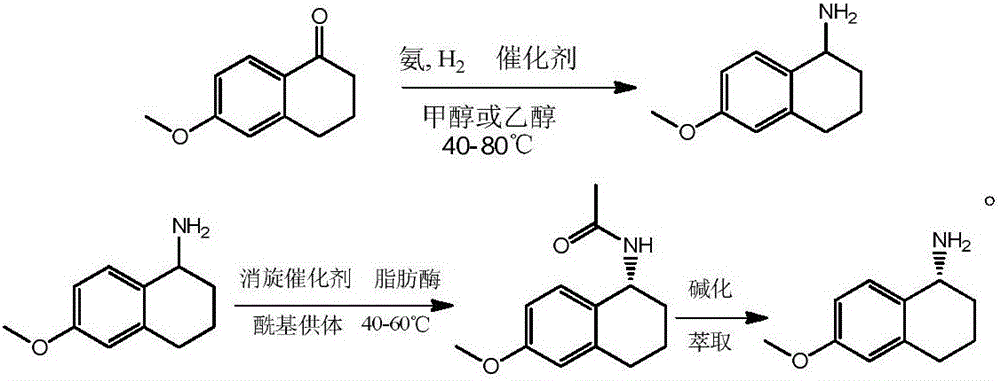
Preparation method of R-7-chloro-1-tetralinylamine
InactiveCN106381324AEasy to operateHigh yieldPreparation by reductive alkylationFermentationKinetic resolutionRacemization
The invention discloses a preparation method of R-7-chloro-1-tetralinylamine. The method comprises the following steps: carrying out reduction ammonification reaction on the raw material 7-chloro-1-tetralone to obtain 7-chloro-1,2,3,4-tetrahydronaphthyl-1-amine, and carrying out dynamic kinetic resolution on the 7-chloro-1,2,3,4-tetrahydronaphthyl-1-amine by using the combination of lipase and a racemization catalyst to obtain the R-7-chloro-1,2,3,4-tetrahydronaphthyl-1-amine. The method is simple to operate, and has the characteristics of wide raw material sources, favorable product yield, high optical purity of the resolution product and the like.
Owner:王际菊
A kind of method of asymmetric catalytic synthesis (r)-4,7-dimethyl-1-tetralone
The invention discloses a method for asymmetrically catalyzing and synthesizing (R)-4, 7-dimethyl-1-tetralone. According to the method, an asymmetrical Kumada cross coupling reaction is conducted on racemization 2-halogenated propionate ester catalyzed by bis oxazoline / cobalt and a methyl phenyl grignard reagent, and (S)-P-toluene propionate ester 2 is firstly generated; then, (S)-P-toluene propionate ester 2 is reduced to (S)-P-toluene propyl alcohol 3 through diisobutylaluminium hydride (DIBAL-H), and then (R)-4-p-methylphenyl-1-amylene 5 is obtained through bromine generation and coupling with and vinyl grignard reagent; next, a hydroboration-oxidation reaction and a Dess-Martin oxidizing reaction are sequentially conducted, and (R)-4-p-methylphenyl valeraldehyde 6 is obtained; finally, oxidation is conducted through silver oxide, an intramolecular Fourier acyl reaction is conducted, and (R)-4,7-dimethyl-1-tetralone is obtained in a ring-closure synthesis mode. The synthesis route is simple and concise, 8 reactions are conducted in all, the total yield is 27%, and the optical purity of a product is 90%.
Owner:CHINA AGRI UNIV
Method for separating and purifying 4,8-dht from pecan exocarp and application of 4,8-dht
ActiveCN104292092BInhibition of germinationGrowth inhibitionBiocidePlant growth regulatorsGradient elutionSilica gel
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Synthesis and resolution of 6-methoxy-1-aminotetralin
The invention discloses a method for preparing racemic 6-methoxy-1-aminotetralin and R-6-methoxy-1-aminotetralin with 6-methoxy-1-tetralone as the raw material. The method specifically includes the steps that 6-methoxy-1-tetralone is subjected to a reductive ammoniation reaction to obtain racemic 6-methoxy-1-aminotetralin, and racemic 6-methoxy-1-aminotetralin is subjected to dynamic kinetic resolution to obtain R-6-methoxy-1-aminotetralin. The method has the advantages that operation is easy, the product yield is high, and the optical purity is high. The method has great guidance and application value in the synthesis and resolution process of 6-methoxy-1-aminotetralin.
Owner:王际宽
Preparation method of 3,4-cyclopentyl-1-tetralone
ActiveCN105936623AEfficient constructionHigh reaction yieldOrganic compound preparationCarboxylic acid esters preparationBenzaldehydeNitrogen
The invention discloses a preparation method of 3,4-cyclopentyl-1-tetralone; under a condition of the presence of a copper catalyst, a nitrogen ligand, a reducing agent and an alkali, alkenyl benzaldehyde and an alpha-brominated unsaturated carbonyl compound are stirred in a solvent and subjected to a reaction for 12 hours at room temperature, and the 3,4-cyclopentyl-1-tetralone is obtained after postprocessing. The method is simple to operate, mild in condition, good in functional group compatibility, wide in substrate applicability, high in yield and good in selectivity, and only needs one-step operation.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method for synthesizing saprorthoquinone
The invention belongs to the field of chemical synthesis, and in particular relates to a method for synthesizing saprorthoquinone which is shown as a formula (I) and serves as a natural product with high antitumor activity. The method comprises the following steps of: performing carbonyl alpha-site methylation, selective isopropyl substitution of an aromatic ring and Grignard reaction on 7-methoxyl-1-tetralone serving as an initiative raw material to prepare a key intermediate, namely 1-(4-methyl-3-pentenyl)-2-methyl-6-isopropyl-7-methoxyl-3,4-dihydronaphthalene; and aromatizing with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ), demethylating with ethanethiol and oxidizing with 2-iodoxybenzoic acid (IBX) to prepare the saprorthoquinone.
Owner:CHINA PHARM UNIV
Method for supercritical separation and purification of 4.8-DHT from pecan epicarp
ActiveCN104292093AInhibition of germinationGrowth inhibitionPlant growth regulatorsCarbonyl compound separation/purificationPlanting seedSolvent
The invention discloses a method for supercritical separation and purification of 4.8-DHT from pecan epicarp. The method comprises the following steps: extracting through a supercritical extraction kettle to obtain an extract; absorbing the extract by using methanol; carrying out silicagel column chromatography; carrying out gradient eluting by using a mixed solvent of petroleum ether and ethyl acetate; collecting a bottle of fluid per column volume, collecting 10 bottles of fluid in all; carrying out reduced-pressure concentration on the fourth bottle of fluid; and crystallizing by using ethyl acetate to obtain a 4,8-dyhydroxy-1-tetralone crystal compound. The 4,8-DHT is a 1:1 raceme and low in impurity content; the purity is 99.82%; the extraction rate is over 60%; and the 4.8-DHT raceme has the effects of inhibiting plant seed germination and seedling growth.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
1-tetralone spirocyclodiene, its synthesis method and its application
InactiveCN105884600BGroup 4/14 element organic compoundsOrganic compound preparationCrotonaldehydeSynthesis methods
The invention relates to 1-tetralone spiro-diene as well as a synthetic method and an application thereof. 1-tetralone spiro-diene is represented by formula (1), and the compound can be used for synthesizing more types of 1-tetralone spiro-framework compounds. 1-tetralone spiro-diene is prepared by the steps of adding 2-crotonaldehyde, tert-butyldimethylsilyl triflate and triethylamine into dichloromethane for reaction so as to obtain a reaction system containing tert-butyl dimethyl silicon protected diene, adding 2-methylene-1-tetralone and zinc trifluoromethanesulfonate into a reaction container, adding an aromatic hydrocarbon solvent under the protection of inert gas, and adding prepared tert-butyl dimethyl silicon protected diene for reaction, so as to finally obtain 1-tetralone spiro-diene. A preparation method of 1-tetralone spiro-diene is simple, the cost is low, and the consumption of catalysts is low.
Owner:ANYANG INST OF TECH
Synthesis method of 2-tetralone derivative
InactiveCN101863769BLow costMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventSynthesis methods
The invention discloses a synthesis method of 1-tetralone derivative, which comprises the step of: in the environment of organic solvent and alkali, leading a compound with a structure of formula 1 and a compound with a structure of formula 2 to have cascade reaction under the action of ligand and catalyst. The synthesis method has raw materials with lower price, is low in cost and mild in reaction condition, causes no environmental pollution, and has the highest yield of 98%.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000012.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000031.PNG)
![Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide Preparing method of N-[2-(7- anisyl-1- naphthyl) ethide] acetamide](https://images-eureka.patsnap.com/patent_img/304a2a1f-93bf-4b9b-8f00-84c91fb25cf8/G2009101403386D0000051.PNG)