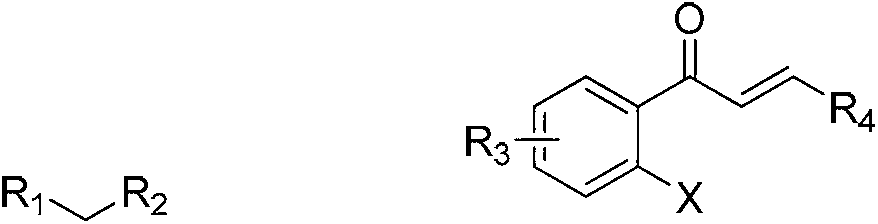
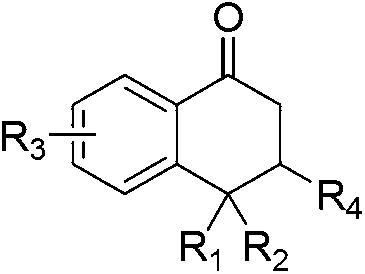
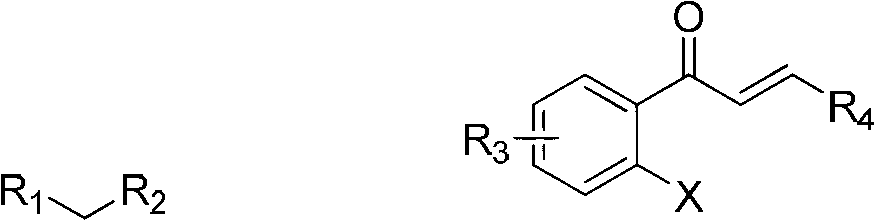Synthesis method of 2-tetralone derivative
A technology of tetralone and a synthetic method, which is applied in the chemical field, can solve problems such as high cost, expensive raw materials, and environmental pollution, and achieve the effects of high yield, low cost, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The reaction formula of the present embodiment is:
[0028]
[0029] In a reaction tube sealed at one end, add 364 mg of (E)-1-(2-iodophenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (MW=364, 1.0 mmol), 480mg diethyl malonate (MW=160, 3mmol), then add 160mg NaOH (MW=40, 4mmol), 26mg L-hydroxyproline (MW=130, 0.2mmol), 19mg CuI (MW =190, 0.1mmol), 2.0mlDMSO as a solvent, under the protection of argon, the time of the series reaction is to stir and react at 20°C for 24h, dilute the reaction mixture with 10ml of water, extract twice with 2×10ml of ethyl acetate, The organic phases were combined, dried and spin-dried under reduced pressure to obtain a crude product. Column chromatography of petroleum ether: ethyl acetate = 10:1 gave 398 mg of the product, 1-tetralone derivative, with a yield of 98%.
[0030] The spectrum of the product: 1H NMR (CDCl3, 400MHz) δ8.13 (d, J=8.0Hz, 1H), 7.72 (d, J=8.0Hz, 1H), 7.63 (t, J=7.6Hz, 1H), 7.49(t, J=7.6Hz, 1H), 7.01(d, J=7.6Hz, 2H), 4.2...
Embodiment 2
[0032]
[0033] In a reaction tube sealed at one end, add 334mg (E)-1-(2-iodophenyl)-3-phenylprop-2-en-1-one (MW=364, 1.0mmol), 226mg cyanoacetic acid Ethyl ester (MW=113, 2mmol); then add 652mg Cs2CO3 (MW=325, 2mmol), 36mg 2-pyridinecarboxylic acid (MW=123, 0.3mmol), 14mg CuBr (MW=141, 0.1mmol), 3.0ml DMSO As a solvent, under the protection of nitrogen, the time for the series reaction was to stir the reaction at 30° C. for 18 hours; the other steps were the same as in Example 1, and finally 310 mg of the product 1-tetralone derivative was obtained, with a yield of 95%.
[0034] Product spectrum: 1H NMR (CDCl3, 400MHz) δ8.15 (d, J=8.0Hz, 1H), 7.74 (d, J=8.0Hz, 1H), 7.64 (t, J=8.4Hz, 1H), 7.51 (t, J=7.6Hz, 1H), 7.19-7.16(m, 3H), 7.09-7.06(m, 2H), 4.32(t, J=5.6Hz, 1H), 4.23-4.10(m, 2H), 3.56(dd, J=5.6Hz, J=17.2Hz, 1H), 3.19(dd, J=6.4Hz, J=17.2Hz, 1H), 1.20(t, J=7.2Hz, 3H), 13C NMR (CDCl3 , 75MHz) δ196.0, 169.6, 168.5, 139.8, 137.8, 133.3, 132.6, 131.9, 128.8, 128.5, 128.3,...
Embodiment 3
[0036]
[0037] In a reaction tube sealed at one end, add 364mg (E)-1-(2-iodophenyl)-3-(3-methoxyphenyl)prop-2-en-1-one (MW=364, 1.0 mmol), 195mg ethyl acetoacetate (MW=130, 1.5mmol), then add 120mg NaOH (MW=40, 3mmol), 52mgL-hydroxyproline (MW=130, 0.4mmol), 19mg CuI (MW=190 , 0.1mmol), 4.0ml DMSO as a solvent, under the protection of nitrogen, the time of the series reaction is to stir the reaction at 35°C for 18h, dilute the reaction mixture with 10ml of water, extract twice with 2×10ml of ethyl acetate, combine the organic phase, dried and spin-dried under reduced pressure to obtain a crude product, petroleum ether: ethyl acetate = 10:1 column chromatography to obtain 360 mg of the product, namely 1-tetralone derivative, with a yield of 98%.
[0038]The spectrum of the product: 1H NMR (CDCl3, 400MHz) δ8.14 (d, J=7.6Hz, 1H), 7.74 (d, J=8.0Hz, 1H), 7.63 (t, J=7.2Hz, 1H), 7.49(t, J=7.6Hz, 1H), 7.08(t, J=8.0Hz, 1H), 6.72(d, J=6.4Hz, 1H), 6.65-6.62(m, 2H), 4.29(t, J =6.0Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com