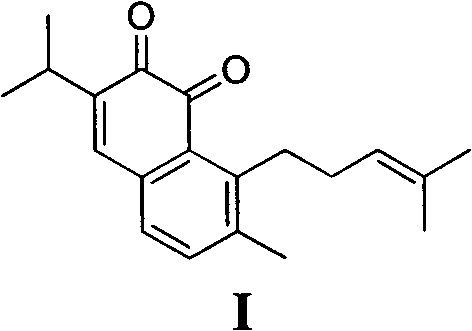
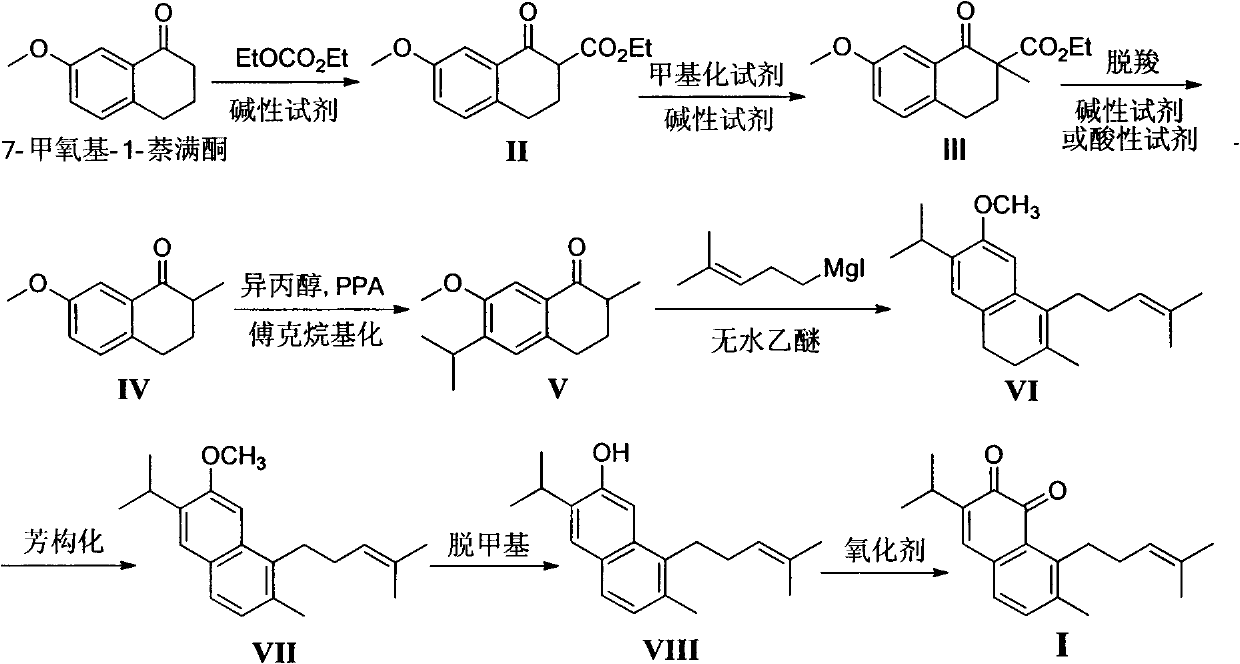
Method for synthesizing saprorthoquinone
A synthesis method and a technology of red root grass, applied in the field of synthesis of natural product red root grass o-quinone, can solve the problems of difficult large-scale production, low reaction yield, harsh reaction conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of ethyl 7-methoxy-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (II):
[0058] Add 20 mL of anhydrous THF to 60% NaH (2.37 g, 0.10 mol), stir for 10 minutes, add diethyl carbonate (6.28 g, 50 mmol), raise the temperature to 50 ° C, slowly dropwise add 7-methoxy-1- A solution made of tetralone (4.75g, 26mol) and 15mL of anhydrous THF was dropped within 30 minutes, refluxed for 5 hours, cooled, and glacial acetic acid was added dropwise in an ice bath to adjust the pH to 5. Add 80mL of water and stir for 30 minutes. Minutes, separate the organic layer, extract the aqueous layer with ethyl acetate, combine the organic phases, wash with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to obtain 6.52 g of oily liquid, which is directly put into the next reaction. The sample required for analysis can be obtained by column chromatography (petroleum ether) to obtain a white solid, m.p.40-42°C. Compound (II) is known, and its CA...
Embodiment 2
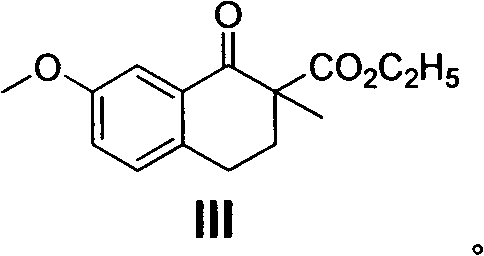
[0060] Preparation of ethyl 2-methyl-7-methoxy-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate (III):
[0061] Dissolve ethyl 7-methoxy-1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate from the previous step in 50 mL of acetone, then add K 2 CO 3 (9g, 65mmol) and CH 3 I (3.20g, 28mmol), reflux reaction 5 hours, cooling, filter excess K 2 CO 3 , and concentrated under reduced pressure to obtain 7.02 g of light yellow oil, which was directly put into the next reaction. The samples required for analysis can be obtained as colorless oil by column chromatography (petroleum ether).
[0062] ESI-MS: 263[M+H] + ;
[0063] 1 H-NMR (300MHz, CDCl 3 ), δ(ppm): 1.29(t, J=8.0Hz, 3H), 1.56(s, 3H), 2.20-2.25(m, 2H), 2.75-2.85(m, 2H), 3.83(s, 3H) , 4.21(q, J=8.0Hz, 2H), 7.12(d, J=7.5Hz, 1H), 7.26(d, J=7.5Hz, 1H), 7.47(s, 1H).
Embodiment 3
[0065] Preparation of 2-methyl-7-methoxy-1-tetralone (IV):
[0066] Decarboxylation under alkaline reagent: Dissolve the oil obtained in Example 2 in 50 mL of ethanol and 10 mL of water, add KOH (4.38 g, 78 mmol), reflux for 8 hours, add 200 mL of water, stir for 15 minutes, dichloromethane extraction, Washed with 5% hydrochloric acid to neutral, washed with saturated brine, anhydrous Na 2 SO 4 Drying, silica gel column chromatography (petroleum ether) gave 4.0 g of a light yellow oily liquid, and the total yield of the above three-step reaction was 78% (calculated as 7-methoxy-1-tetralone).
[0067] Decarboxylation under acidic reagent: dissolve the oil obtained in Example 2 in 150 mL of glacial acetic acid, add 15 mL of concentrated hydrochloric acid, reflux for 4 hours, add 200 mL of water, stir for 10 minutes, extract with dichloromethane, and wash with saturated sodium carbonate solution until Neutral, washed with saturated brine, anhydrous Na 2 SO 4 Drying, silica ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com