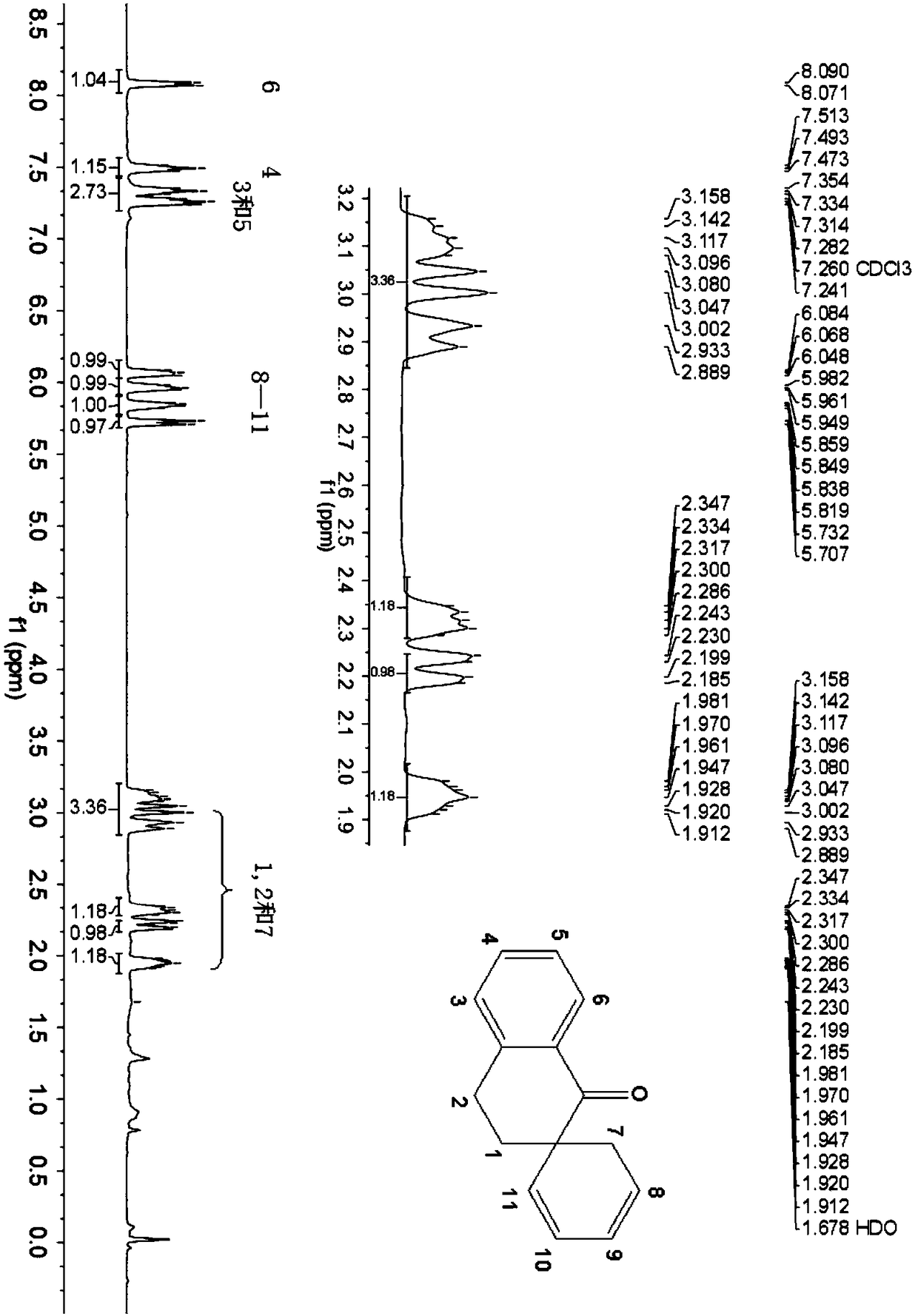
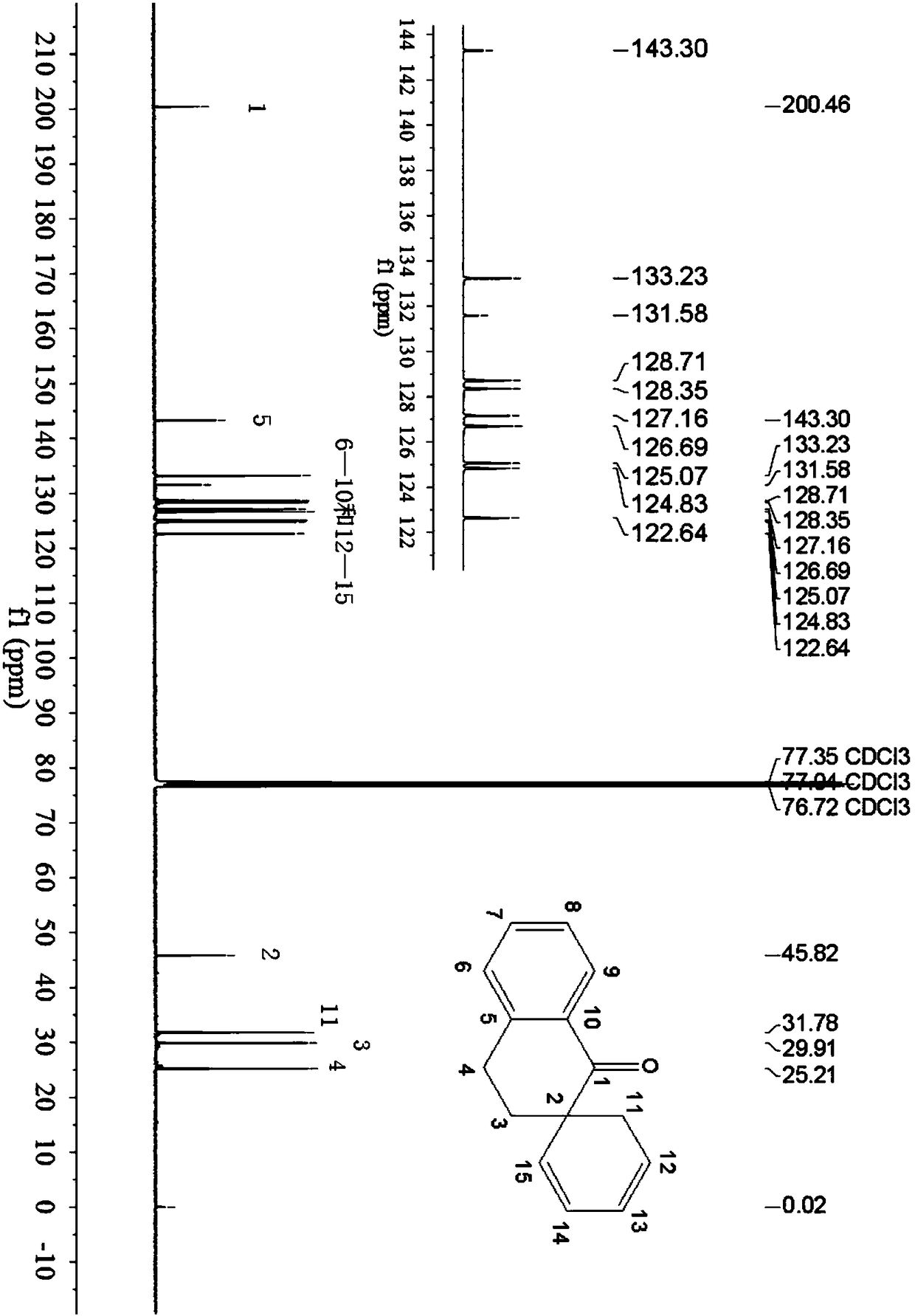
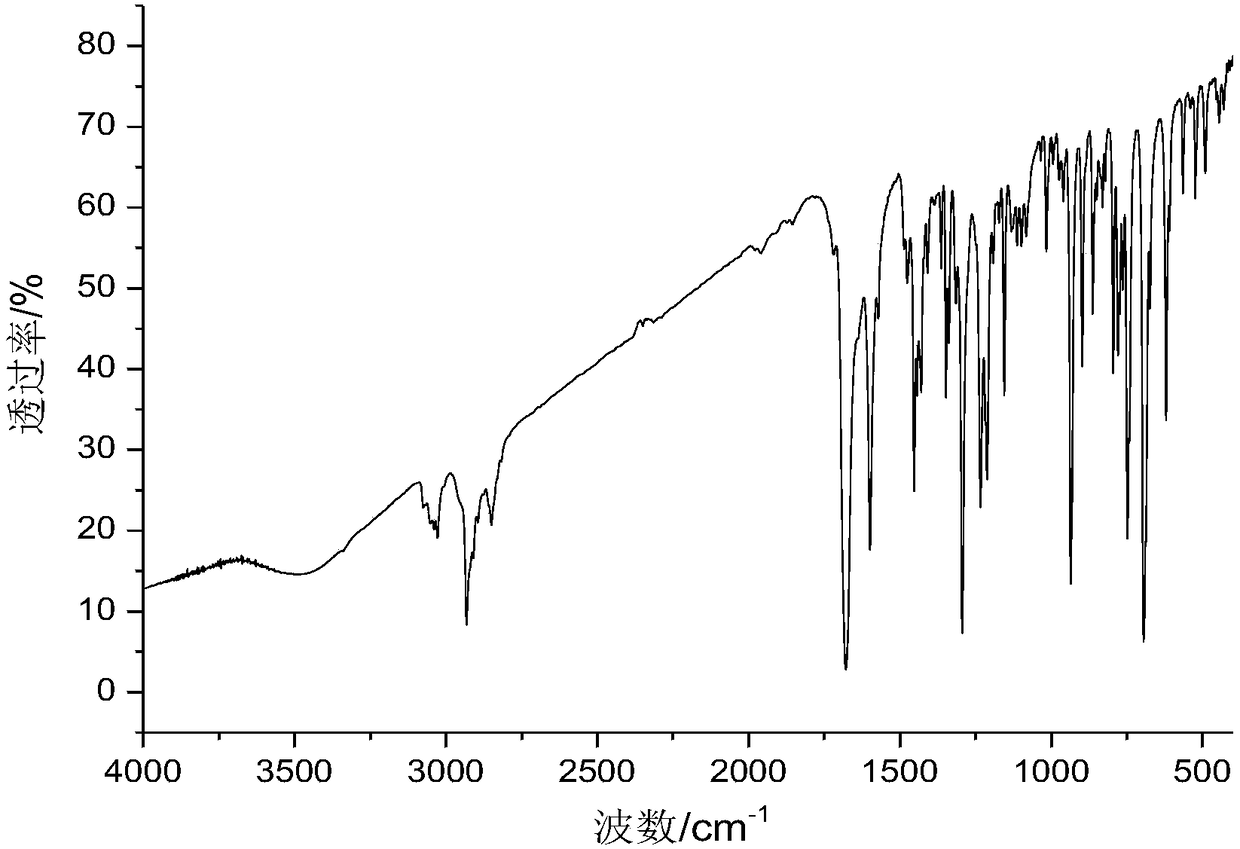
1-tetralone spirocyclodiene, its synthesis method and its application
A kind of technology of tetralinone spiro and tetralinone, applied in chemical instrument and method, compound of periodic table 4/14 group element, carbon-based compound preparation and other directions, can solve the problem of 1-tetralinone spiro that has not yet been reported in the literature. The problems such as the synthesis method of cyclodiene, to achieve the effects of being conducive to large-scale production and promotion, the preparation method is simple, and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The preparation of embodiment 1 1-tetralone spiro diene
[0049] (1) Add 5mmol of 2-butenal, 5.5mmol of tert-butyldimethyltrifluoromethanesulfonate and 7.5mmol of triethylamine in a 100mL round-bottomed flask, then add 15mL of dichloromethane, at 45°C Under reaction for 48 hours, after the end of the reaction, add saturated aqueous solution of sodium bicarbonate to quench the reaction, then wash with water, then wash with saturated brine, wherein the volume of saturated aqueous solution of sodium bicarbonate, water and brine is 10mL, then the organic phase is directly Spin-dried to obtain the tert-butylsilyl-protected diene shown in the following formula, with a calculated yield of 96%;
[0050]
[0051] (2) 5mmol 2-methylene-1-tetralone and 1mmol zinc trifluoromethanesulfonate are added to the reaction tube, and the nitrogen is pumped through the double row tube for three times, then 15mL of toluene is added, and then 10mmol of the step ( The tert-butyldimethylsily...
Embodiment 2
[0052] The preparation of embodiment 2 1-tetralone spiro diene
[0053] (1) In a 100mL round bottom flask, add 10mmol of 2-butenal, 12mmol of tert-butyldimethyltrifluoromethanesulfonate and 20mmol of triethylamine respectively, then add 30mL of dichloromethane, at 50°C Reacted for 42 hours. After the reaction was over, add a saturated aqueous solution of sodium bicarbonate to quench the reaction, then wash with water, and then wash with saturated brine, wherein the saturated aqueous solution of sodium bicarbonate used, the volume of water and brine is 30mL, and then the organic phase is directly Spin-dried to obtain the tert-butylsilyl-protected diene shown in the following formula, with a calculated yield of 95%;
[0054]
[0055] (2) Add 5mmol 2-methylene-1-tetralone and 0.75mmol zinc trifluoromethanesulfonate into the reaction tube, pump and change nitrogen three times through the double row tube, then add 15mL toluene, and then add 12.5mmol The tert-butyldimethylsilyl-...
Embodiment 3
[0056] Example 3 Preparation of 1-tetralone spirodiene
[0057] (1) In a 100mL round bottom flask, add 15mmol of 2-butenal, 19.5mmol of tert-butyldimethyltrifluoromethanesulfonate and 37.5mmol of triethylamine, then add 45mL of dichloromethane, at 60 ℃ for 36 hours, after the reaction, quench the reaction by adding saturated aqueous solution of sodium bicarbonate, then wash with water, and then wash with saturated brine, wherein the volumes of saturated aqueous solution of sodium bicarbonate, water and brine are 60mL, and then the organic phase Spin dry directly to obtain the tert-butyldimethylsilyl-protected diene shown in the following formula, with a calculated yield of 94%;
[0058]
[0059] (2) Add 5mmol 2-methylene-1-tetralone and 0.5mmol zinc trifluoromethanesulfonate into the reaction tube, pump and change nitrogen three times through the double row tube, then add 15mL toluene, and finally add 15mmol step The tert-butyldimethylsilyl-protected diene obtained in (1) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com