1,3-butanediol synthesis method and system
A synthesis method and technology of butanediol, applied in chemical instruments and methods, preparation of heterocyclic compounds, preparation of hydroxyl compounds, etc., can solve the problems affecting product purity, odor, chroma, high viscosity of 3-hydroxybutyraldehyde, Color and taste indicators are unqualified and other problems, so as to improve the utilization rate of raw materials, reduce back-mixing, and improve stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] A kind of synthetic method of 1,3-butanediol (1,3-BDO) provided by one aspect of the embodiment of the present invention, it comprises:
[0042] The condensation reaction system containing uniformly mixed acetaldehyde and alkali catalyst is carried out at 0-20°C for 11min-24h under the condition of pH value of 9-12.8, and the obtained product mainly contains 2,6-dimethyl- Condensation products of 1,3-dioxan-4-ol;
[0043] The cleavage reaction system comprising the 2,6-dimethyl-1,3-dioxan-4-ol and the cleavage catalyst is subjected to a cleavage reaction at 50-110°C for 1min-5h to obtain a Cleavage products of polybutyraldehyde;
[0044]Under the condition that the hydrogenation pressure is 1-15 MPa, the hydrogenation reaction system containing the dimer butanol aldehyde and the hydrogenation catalyst is subjected to ring-opening hydrogenation reaction at 60-180° C. for 0.2-20 hours to obtain 1, 3-butanediol.
[0045] Further, the condensation reaction is carried out...
Embodiment 1
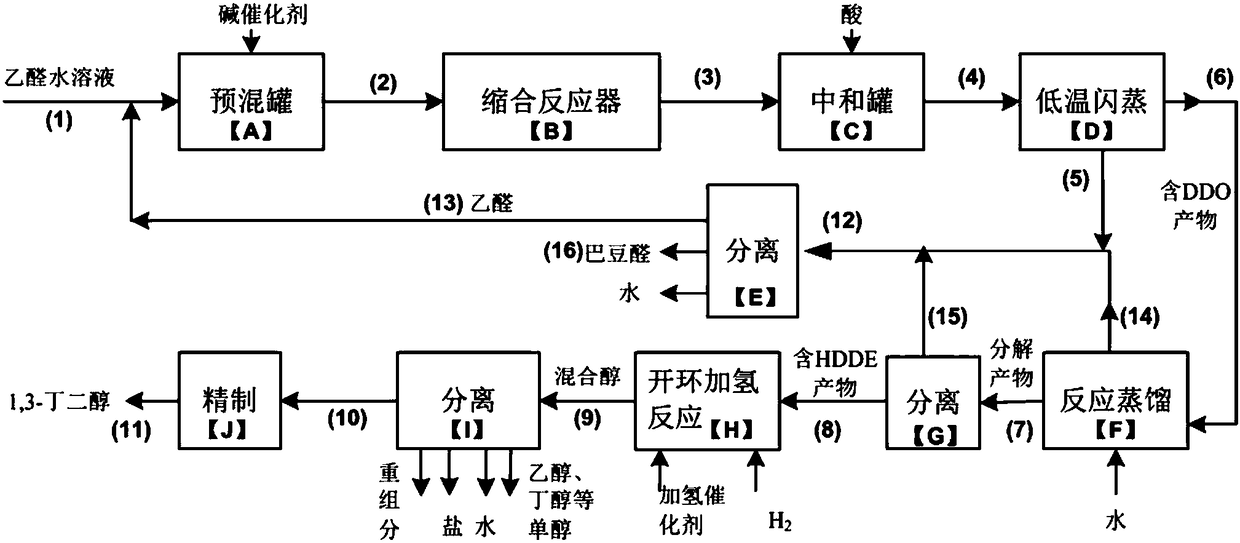
[0096] Example 1 see figure 1 As shown, this embodiment is a specific implementation example of a process flow system for producing 1,3-butanediol from acetaldehyde.
[0097] Specifically, the process can include:
[0098] The aqueous solution containing 85% acetaldehyde and the alkali catalyst are mixed into the shell-and-tube condensation reactor with a built-in spiral member for reaction. The acid content in the raw material acetaldehyde is controlled below 0.05wt%, and the alkali catalyst used is NaOH, NaOH 2 CO 3 、Na 3 PO 4 The pH value of the reaction system was 10.1, the reaction time was 10 h, and the reaction temperature was controlled at 10°C. The outside of the tubular reactor is a circulating water cooling bath, which is counter-currently cooled, that is, the flow direction of the reactants inside the tube is opposite to the flow direction of the cooling water, and 2,6-dimethyl-1,3-dioxane is obtained after the reaction Hexan-4-ol-based product. The condensa...
Embodiment 2
[0102] The aqueous solution containing 83% acetaldehyde and the alkali catalyst are mixed into the tubular condensation reactor with a built-in spiral member for reaction. The acid content in the raw material acetaldehyde is controlled below 0.05 wt%, and the whole reaction process is carried out under nitrogen protection. The base catalyst used is NaOH, Na 3 PO 4 The mixed solution, the pH value of the reaction system is 10.0, the reaction time is 8h, and the reaction temperature is controlled at 0°C. The outside of the tube-and-tube reactor is a circulating water cooling bath, which is counter-currently cooled, that is, the flow direction of the reactants inside the tube is opposite to the flow direction of the cooling water. After the reaction, 2,6-dimethyl-1,3-dioxa Cyclohexane-4-ol-based product. The condensation product enters the neutralization tank, and the pH value of the reaction solution is neutralized to 6.2 with 5 wt % phosphoric acid aqueous solution under rap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| iodine adsorption value | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com