Preparation method of 7-hydroxy-1-tetralone
A technology of tetralone and hydroxyl group is applied in the field of preparation of pharmaceutical intermediate 7-hydroxy-1-tetralone, and can solve the problems of extremely high requirements on equipment and operation safety, difficulty in realizing industrialized production, poor selectivity and the like , to achieve the effect of improving environmental friendliness, simplifying operation and meeting quality requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Preparation of 4-(4-methoxyphenyl)-4-oxobutanoic acid
[0034] Put 250ml of nitromethane and 30g of anisole into a 500ml reaction bottle, add 80g of anhydrous aluminum trichloride at a temperature of 0-15°C, keep stirring for 2 hours, then add 25g of succinic anhydride, and react at a temperature of 10-20°C for 12 hours . The reaction solution was added to a system of 600ml water-100ml 30% hydrochloric acid, the temperature was controlled at 0-25°C, and the mixture was kept stirring for 1 hour. Suction filtration and drying yielded 49.1 g of 4-(4-methoxyphenyl)-4-oxobutanoic acid with a yield of 92% and a purity of 99.5% by HPLC.
Embodiment 2
[0035] Example 2 Preparation of 4-(4-methoxyphenyl)-4-oxobutanoic acid
[0036] In a 500ml reaction bottle, put 250ml of dichloromethane and 50g of anisole, add 120g of aluminum tribromide at a temperature of 0-15°C, stir and keep warm for 2h, add 43g of succinic anhydride, and keep the temperature at -5-20°C for 12h. The reaction solution was added to a system of 600ml water-100ml 30% hydrochloric acid, the temperature was controlled at 0-25°C, and the mixture was kept stirring for 1 hour. After suction filtration, the filter cake was dried under reduced pressure at 60°C to obtain 76.5 g of 4-(4-methoxyphenyl)-4-oxobutanoic acid, with a yield of 86% and a purity of 98.7% by HPLC.
Embodiment 3
[0037] Example 3 Preparation of 7-Hydroxy-1-tetralone
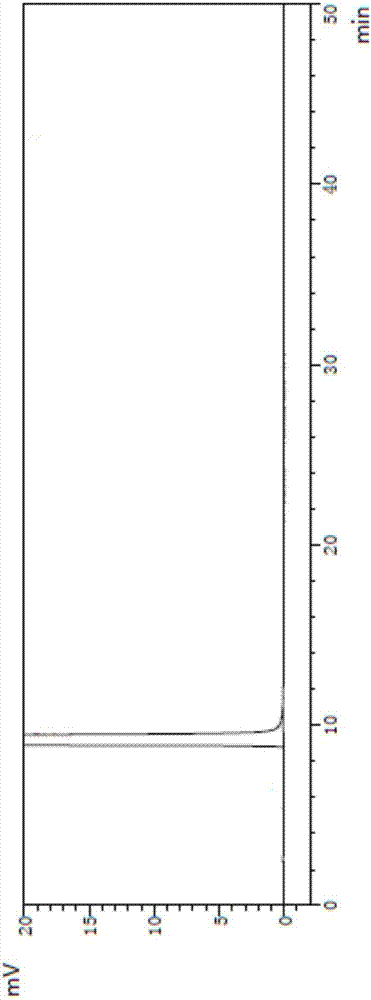
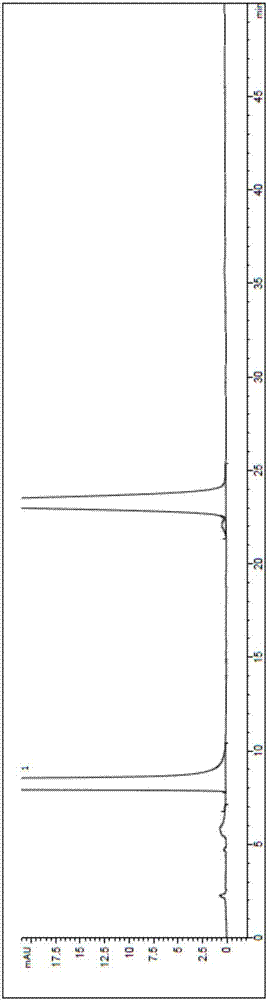
[0038] Put 200ml of toluene, 44g of aluminum trichloride, 10ml of nitromethane into a 500ml reaction bottle, dissolve at 60-70°C, add 4-(4-methoxyphenyl)butyric acid (prepared according to the method described in Example 1) 20g. The temperature was raised to reflux for 10 hours, and the reaction solution was added to 300 ml of ice water. Stand for stratification, discard the water layer, add 100ml of water to the organic layer, adjust the pH to 13 with liquid caustic soda, let stand for stratification, discard the organic layer, adjust the pH of the water layer to 2 with hydrochloric acid, filter, add methanol to the filtrate 20ml and 30ml of water were heated and refluxed to dissolve, cooled to 0°C to crystallize, filtered, and the filter cake was dried to obtain 14.7g of white solid 7-hydroxy-1-tetralone, with a yield of 88%, and a purity of 99.99% by HPLC. See atlas figure 1 .
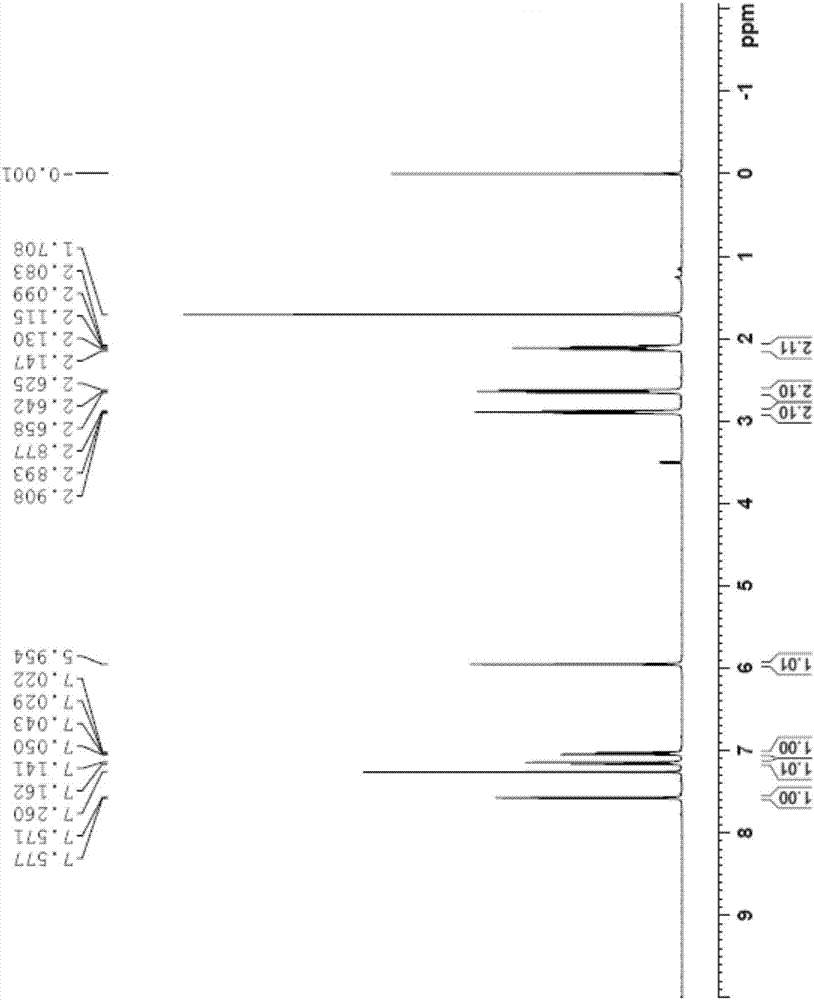
[0039] 1 H-NMR (CDCl 3 ,400MHz)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com