Preparation method of dibucaine hydrochloride
Dibucaine hydrochloride and Dibucaine technology, applied in the field of medicine and chemical industry, can solve problems such as low purity and low yield of Dibucaine hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
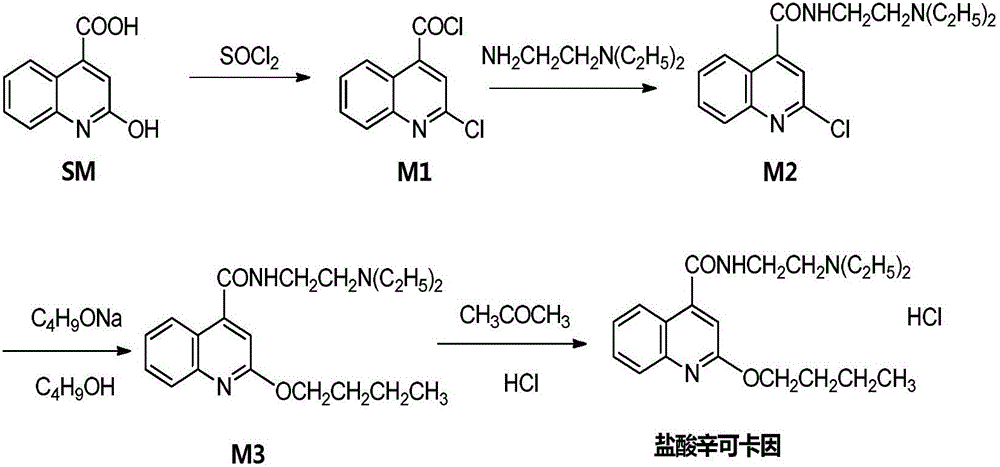
[0060] A. Synthesis of 2-chloro-4-acylchloroquinoline 1
[0061] Add 18.9 grams of 2-hydroxy-4-quinoline carboxylic acid to a 250 ml three-necked flask at room temperature, add 15.3 grams of thionyl chloride dropwise under stirring with 150 ml of toluene, heat up to 60°C for 3 hours, cool to 30°C, Concentrate under reduced pressure, add a little toluene, and continue to concentrate under reduced pressure to dryness. 19.5 g of oil was obtained, yield 91.3%.
[0062] B. Synthesis of 2-chloro-N-[2-(diethylamino)ethyl]-4-quinoline carboxamide
[0063] Dilute the oil obtained above with 200ml of toluene and add it to a 500ml three-necked flask, then add 10g of N,N-diethyldiethylamine, stir at 60°C, cool down to room temperature after the reaction is complete, add water and stir for 30 minutes , liquid separation, the organic layer was washed twice with water, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dried to obtain...
Embodiment 2
[0069] A. Synthesis of 2-chloro-4-acylchloroquinoline
[0070] Add 11 grams of 2-hydroxy-4-quinoline carboxylic acid to a 250 ml three-necked flask at room temperature, add 11.2 grams of thionyl chloride dropwise under stirring with 110 ml of toluene, heat up to 45°C for 2.5 hours, cool to 25°C, reduce Concentrate under reduced pressure, add a little toluene, and continue to concentrate under reduced pressure to dryness to obtain 15.4 g of oil, with a yield of 83.3%;
[0071] B. Synthesis of 2-chloro-N-[2-(diethylamino)ethyl]-4-quinoline carboxamide
[0072] Dilute the oil obtained above with 300 ml of toluene and add it to a 500 ml three-necked flask, then add 7 grams of N,N-diethyldiethylamine, stir at 80°C, cool to room temperature after the reaction is complete, add water and stir for 25 minutes , liquid separation, the organic layer was washed twice with water, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-drie...
Embodiment 3
[0078] A. Synthesis of 2-chloro-4-acylchloroquinoline
[0079]Add 28.9 grams of 2-hydroxy-4-quinoline carboxylic acid to a 250 ml three-necked flask at room temperature, add 19.2 grams of thionyl chloride dropwise under stirring with 190 ml of toluene, heat up to 75°C for 3.5 hours, cool down to 35°C, reduce Concentrate under reduced pressure, add a little toluene, and continue to concentrate under reduced pressure to dryness. 19.5 g of oil was obtained, yield 88.3%.
[0080] B. Synthesis of 2-chloro-N-[2-(diethylamino)ethyl]-4-quinoline carboxamide
[0081] Dilute the oil obtained above with 220ml of toluene and add it to a 500ml three-necked flask, then add 14.5g of N,N-diethyldiethylamine, stir at 70°C, cool down to room temperature after the reaction is complete, add water and stir for 40 minutes , liquid separation, the organic layer was washed twice with water, washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was spin-dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com