Aescine A injection and preparation method therefor
A escin and injection technology, which is applied in the field of pharmaceutical preparations, can solve the problems of inability to reduce nephrotoxicity, and achieve good resolubility and quality stability, no atrophy, and strong anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
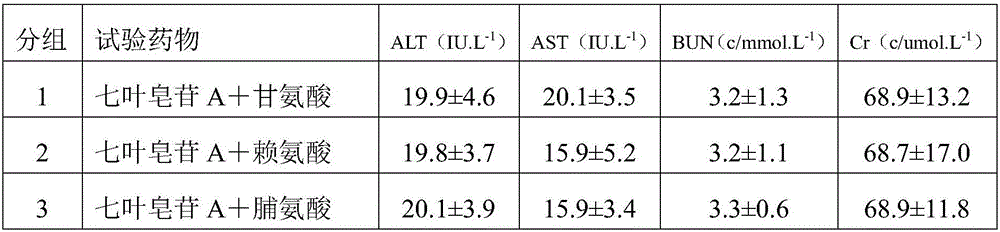
[0029] 1) A mixed solution prepared by dissolving 30g aescin A, 2g glycine, and 200g mannitol in 2000ml water for injection, the concentration of mannitol in the mixed solution is 10g / 100ml, and the concentration of aescin A is 1.5g / 100ml , The concentration of glycine is 0.1g / 100ml;
[0030] 2) Adjust the pH of the mixed solution to 6.5 with lactic acid;
[0031] 3) Filter the pH-adjusted solution with a 0.22μm filter membrane;
[0032] 4) Freeze drying after filling.
[0033] The freeze-drying procedure is as follows:
[0034] Pre-freezing: first quickly reduce the material temperature to -20°C and keep it for 1 hour, then slowly lower the material temperature to -40°C and keep it warm for 4 hours;
[0035] Sublimation at one time: control the vacuum degree below 20 Pa, raise the temperature of the material to -10℃ and keep it for 4 hours;
[0036] Dry again: Raise the temperature of the material to 35°C and keep it for 12 hours.
Embodiment 2
[0038] 1) A mixed solution was prepared by dissolving 40 g aescinate A, 6 g lysine, and 800 g mannitol in 4000 ml water for injection. The concentration of mannitol in the mixed solution was 20 g / 100 ml, and the concentration of aescin A was 1 g / 100ml, the concentration of lysine is 0.15g / 100ml;
[0039] 2) Adjust the pH of the mixed solution to 4.5 with lactic acid;
[0040] 3) Filter the pH-adjusted solution with a 0.22μm filter membrane;
[0041] 4) Freeze drying after filling.
[0042] The freeze-drying procedure is as follows:
[0043] Pre-freezing: first reduce the temperature of the material quickly to -25°C and keep it for 0.5 hours, then slowly reduce the temperature of the material to -35°C and keep it warm for 5 hours;
[0044] One sublimation: control the vacuum degree below 20 Pa, raise the temperature of the material to -5°C and keep it warm for 3 hours;
[0045] Dry again: Raise the temperature of the material to 40°C and keep it for 10 hours.
Embodiment 3
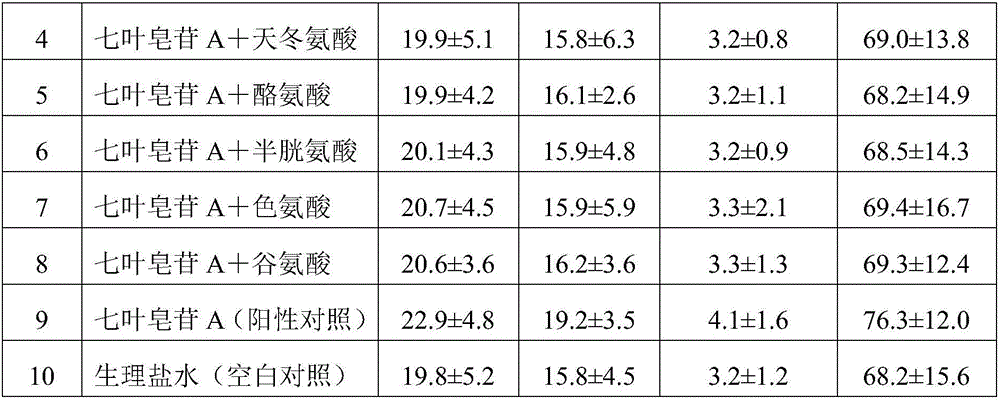
[0047] 1) 25g aescinate A, 5g aspartic acid, 150g mannitol are dissolved in 1250ml water for injection to prepare a mixed solution, the concentration of mannitol in the mixed solution is 12g / 100ml, and the concentration of aescin A is 2g / 100ml, the concentration of aspartic acid is 0.4g / 100ml;
[0048] 2) Adjust the pH of the mixed solution to 5.5 with lactic acid;
[0049] 3) Filter the pH-adjusted solution with a 0.22μm filter membrane;
[0050] 4) Freeze drying after filling.
[0051] The freeze-drying procedure is as follows:
[0052] Pre-freezing: first quickly reduce the material temperature to -22°C and keep it for 0.5 hours, then slowly reduce the material temperature to -38°C and keep it warm for 3 hours;
[0053] Sublimation once: control the vacuum degree below 20 Pa, raise the temperature of the material to -15℃ and keep it for 5 hours;
[0054] Dry again: Raise the temperature of the material to 30°C and keep it warm for 15 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com