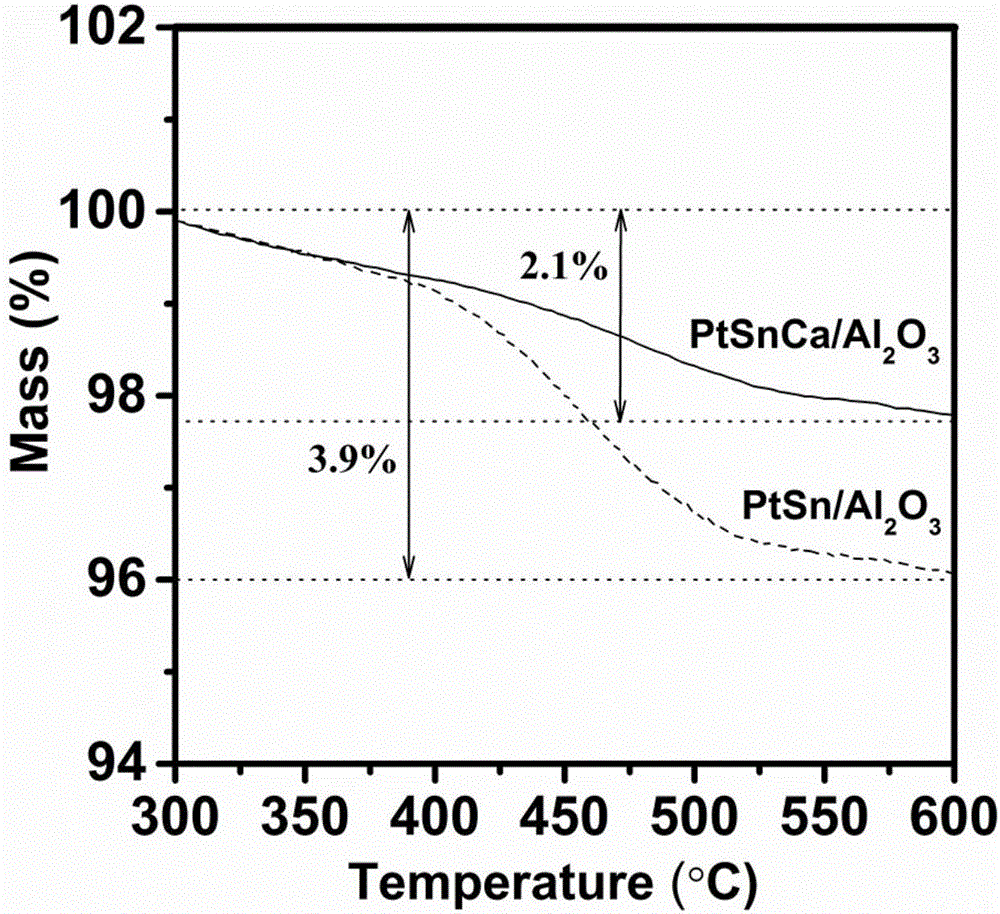
Anti-carbon deposition platinum-based catalyst for preparing propylene through propane dehydrogenation and preparation method thereof
A carbon-deposition platinum-based propane and catalyst technology, which is applied in the field of carbon-deposition-resistant platinum-based propane dehydrogenation to propylene catalysts and its preparation, can solve problems such as poor stability and easy carbon deposition and deactivation of catalysts, and achieve enhanced stability and reduced The effect of carbon deposition rate and carbon deposition reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take 400μL 0.05g / mL Ca(NO 3 ) 2 Ethanol solution impregnated to 1g Al 2 o 3 On the carrier, let it stand at room temperature for 2h, and dry it overnight at 50°C, and the obtained sample was stored in 20% O 2 / N 2 Calcined at 500℃ for 4h in atmosphere; 13.27mg H 2 PtCl 6 ·6H 2 O and 14.94 mg SnCl 2 2H2 The O precursor was dissolved into 330 μL ethanol solution, and an equal volume of the above solution was impregnated into 1 g Al 2 o 3 - In the CaO carrier, the sample was left at room temperature for 2 hours after impregnation, and dried overnight in a 50°C oven. 2 / N 2 Atmosphere, calcination at 500°C for 4h, then in 20%H 2 / N 2 Atmosphere, 590°C reduction for 2 hours, the obtained catalyst sample is marked as A, and the evaluation results are shown in Table 1.
Embodiment 2
[0034] 24.60mg Ca(NO 3 ) 2 and 13.52 mg SnCl 2 2H 2 O dissolved in 300 μL water and impregnated to 1 g Al 2 o 3 On the carrier, let it stand at room temperature for 2h, and dry it overnight at 50°C, and the obtained sample was stored in 20% O 2 / N 2 Calcined at 500°C for 4h in the atmosphere; then 15.47mgH 2 PtCl 6 ·6H 2 The O precursor was dissolved into 330 μL water, and an equal volume of the solution was impregnated into 1 g Al 2 o 3 -CaO-SnO 2 In the carrier, the sample was left at room temperature for 2 hours after impregnation, and dried overnight in a 50°C oven. 2 / N 2 Atmosphere, calcination at 500°C for 4h, then in 20%H 2 / N 2 Atmosphere, 600°C reduction for 2h, the obtained catalyst sample is marked as B, and the evaluation results are shown in Table 1.
[0035] Table 1
[0036]
Embodiment 3
[0038] 32.80mg Ca(NO 3 ) 2 Dissolved in 300 μL water and impregnated to 1 g Al 2 o 3 On the carrier, let it stand at room temperature for 2h, and dry it overnight at 50°C, and the obtained sample was stored in 20% O 2 / N 2 Calcined at 500°C for 4h in the atmosphere; 14.94mg SnCl 2 2H 2 O dissolved in 300 μL water and impregnated to 1 g Al 2 o 3 - on the CaO carrier, let it stand at room temperature for 2h, and dry it in a 50°C oven overnight. 2 / N 2 In the atmosphere, calcined at 500°C for 4h; then 13.27mg H 2 PtCl 6 ·6H 2 The O precursor was dissolved into 330 μL water, and an equal volume of the solution was impregnated into 1 g Al 2 o 3 -CaO-SnO 2 In the carrier, the sample was left at room temperature for 2 hours after impregnation, and dried overnight in a 50°C oven. 2 / N 2 Atmosphere, calcination at 500°C for 4h, then in 20%H 2 / N 2 Atmosphere, 620°C reduction for 2h, the obtained catalyst sample is denoted as C, and the evaluation results are shown in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com