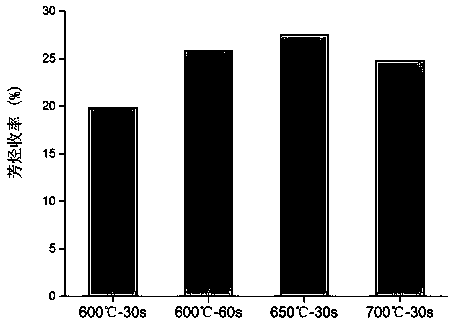A kind of method for preparing aromatic hydrocarbon by catalytic pyrolysis of waste polyolefin plastic
A technology of catalytic pyrolysis and waste polyolefin, which is applied in the preparation of liquid hydrocarbon mixtures and the petroleum industry to achieve the effects of short reaction cycle, mild reaction conditions and convenient recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Catalytic fast pyrolysis of polypropylene and polyethylene
[0025] Experimental raw materials: polypropylene, polyethylene
[0026] Reaction device: catalytic pyrolysis instrument (CDS5200HP-R analytical pyrolyzer)
[0027] Pyrolysis temperature: 650°C
[0028] Pyrolysis time: 30s
[0029] Reaction pressure: normal pressure
[0030] Heating rate: 20℃ / ms
[0031] Catalyst: niobic acid (purity>99%)
[0032] Product analysis: Agilent6890 / 5973i GC / MS.
[0033] Note: The reaction carrier gas is helium; the yield is calculated based on the molar carbon yield of the product.
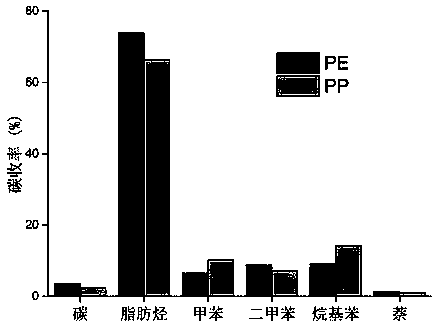
[0034] Table 1 Yields of CFP reaction products of different raw materials
[0035]
[0036] Note: a Including ortho, meta and para xylenes;
[0037] b Includes isomers of ethylbenzene, methylethylbenzene and mesitylene;
[0038] c Including naphthalene, alkyl naphthalene.
[0039] The present embodiment obtains the yield of each product as figure 1 shown.
Embodiment 2
[0040] Example 2 Effect of pyrolysis temperature and pyrolysis time on catalytic fast pyrolysis
[0041] Experimental raw material: polyethylene
[0042] Reaction device: catalytic pyrolysis instrument (CDS5200HP-R analytical pyrolyzer)
[0043] Pyrolysis temperature: 600°C, 650°C, 700°C
[0044] Pyrolysis time: 30s, 60s
[0045] Reaction pressure: normal pressure
[0046] Heating rate: 20℃ / ms
[0047] Catalyst: niobic acid (purity>99%)
[0048] Product analysis: Agilent6890 / 5973i GC / MS.
[0049] Note: The reaction carrier gas is helium; the yield is calculated based on the molar carbon yield of the product.
[0050] In order to study the suitable pyrolysis temperature and time of polyolefin plastic raw materials, polyethylene was used as the reaction raw material and niobic acid was used as the catalyst to evaluate the catalytic reaction, see figure 2 . From figure 2 It can be seen that the yield of aromatics is the lowest when the pyrolysis temperature and time ar...
Embodiment 3
[0051] Example 3 Effect of Phosphoric Acid Modified Niobic Acid on Catalytic Rapid Pyrolysis of Polyolefin Plastics
[0052] Experimental raw material: polypropylene
[0053] Reaction device: catalytic pyrolysis instrument (CDS5200HP-R analytical pyrolyzer)
[0054] Pyrolysis temperature: 600℃
[0055] Pyrolysis time: 60s
[0056] Reaction pressure: normal pressure
[0057] Heating rate: 20℃ / ms
[0058] Catalyst: niobic acid, impregnated with equal volumes of 0.5M / L, 1M / L, 1.5M / L and 2M / L phosphoric acid respectively
[0059] Product analysis: Agilent6890 / 5973i GC / MS.
[0060] Note: The reaction carrier gas is helium; the yield is calculated based on the molar carbon yield of the product.
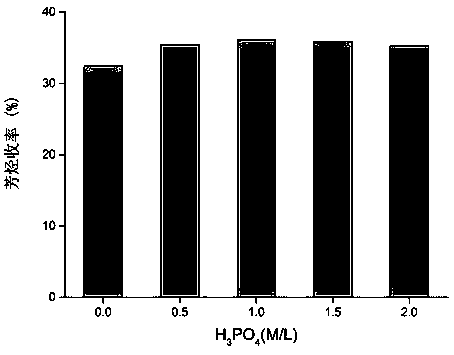
[0061] In order to increase the yield of aromatics in the catalytic pyrolysis reaction of polyolefin plastics, niobic acid was impregnated with phosphoric acid of different molar concentrations, and its catalytic activity was studied, see image 3 . Depend on image 3 It can be seen ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com