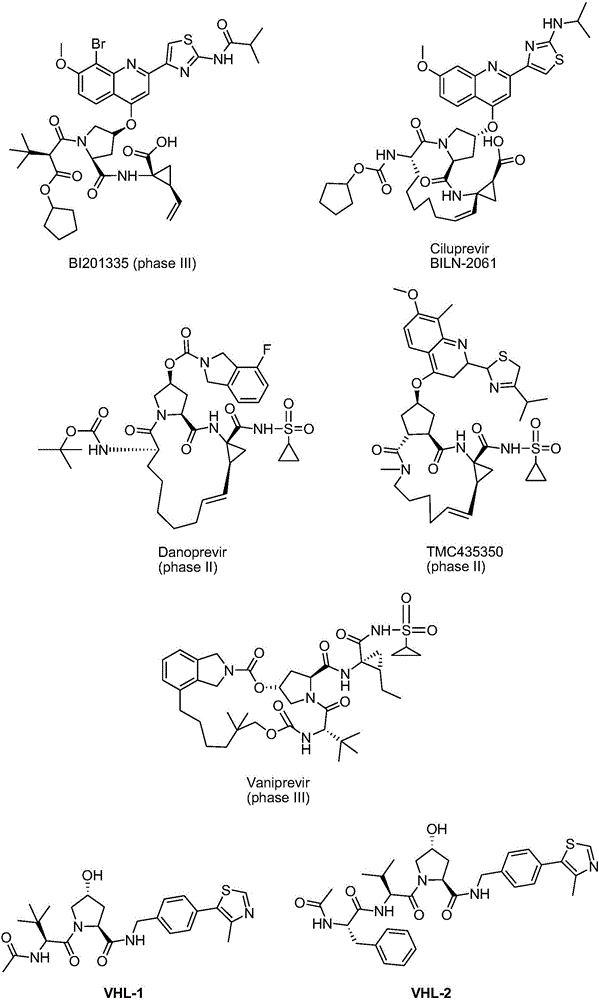
Preparation method for medical intermediate N-Boc-cis-4-hydroxy-L-proline
A technology of n-boc- and intermediates, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of long route, difficult purification, high production cost, etc., and achieve good reaction selectivity, low preparation cost, and high ee value Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The following description serves to disclose the present invention to enable those skilled in the art to carry out the present invention. The preferred embodiments described below are only examples, and those skilled in the art can devise other obvious variations.
[0028] The synthesis method comprises using L-hydroxyproline as a raw material, protecting it with Boc, then oxidizing the hydroxyl group to obtain N-Boc-4-oxo-L-proline, finally dissolving it in a solvent, and reducing it at a certain temperature reagent reduction and purification to obtain the product. This process is shown in Equation 7:
[0029]
[0030] Specific steps are as follows:
[0031] Step 1: Preparation of intermediate 2 (tras-F-1)
[0032] L-Hydroxyproline (65.6 g, 0.5 mol, 1.0 e.q.) was added to NaOH solution (1.0 M, 525 mL, 8.0X) and dioxane (525 mL, 8.0X). Cool down to 0-5C, slowly drop Boc2O (120g, 0.55mol, 1.1e.q.) into it, keep warm at 0-5C and stir after dropping, monitor the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com