Morphine derivative crystal form III, preparation method, and application thereof
A technology of derivatives and crystal forms, applied in the field of crystal form III of morphine derivatives and its preparation, can solve problems such as unstable placement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1 g of the morphine derivative shown in Formula 1 (prepared according to the method described in CN201410116005.0) and 3 ml of acetonitrile were prepared as a suspension, stirred at 5°C for 15 h, stirred and crystallized at 0°C for 10 h, filtered, and dried under reduced pressure at 60°C.
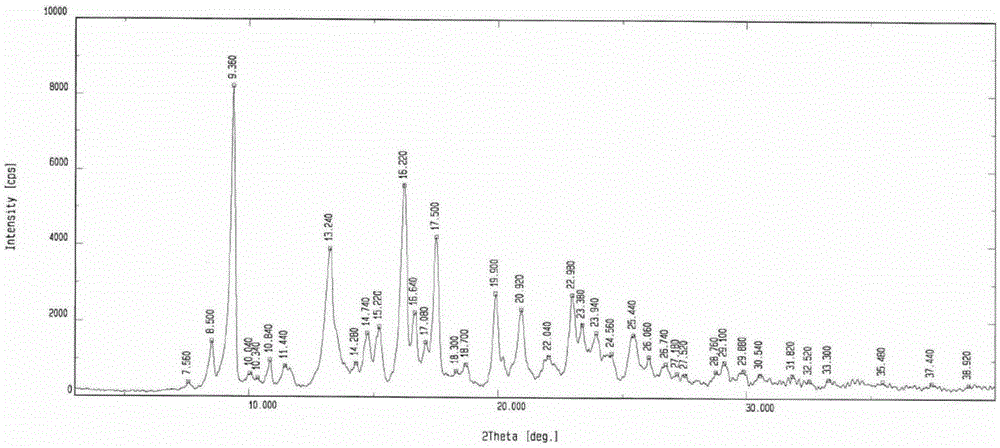
[0070] The obtained morphine derivatives are 7.56, 8.50, 9.36, 10.04, 10.34, 10.84, 11.44, 13.24, 14.28, 14.74, 15.22, 16.22, 16.64, 17.08, 17.50, 18.30, 18.70, 19.90, 20.942, 22. There are absorption peaks at 22.98, 23.38, 23.94, 24.56, 25.44, 26.06, 26.74, 27.18, 27.52, 28.76, 29.10, 29.88, 30.54, 31.82, 32.52, 33.30, 35.48, 37.44, and 38.92. It is crystal form III. Its X powder diffraction pattern is as figure 1 shown.
[0071] Morphine derivative crystal form III shown in crystal form 1 of the present invention, its powder X-ray pattern is represented by 2θ, d-value, I / I 0 Other parameters express the III crystal form, as shown in the table below:
[0072] 2θ° d-va...
Embodiment 2
[0078] Prepare a suspension of 1 g of the morphine derivative shown in Formula 1 and 5 ml of acetonitrile, stir at 10°C for 5 h, stir and crystallize at 5°C for 1 h, filter, and dry under reduced pressure at 50°C, and the obtained morphine derivative is identified by the characterization method in Example 1 , is Form III.
Embodiment 3
[0080] Prepare a suspension of 1 g of the morphine derivative shown in Formula 1 and 10 ml of acetonitrile, stir at 65°C for 11 h, stir and crystallize at 20°C for 15 h, filter, and dry under reduced pressure at 70°C. The resulting morphine derivative is identified by the characterization method in Example 1 , is Form III.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com