Tedizolid impurity and preparing method thereof
A technology of tedizolid and impurities, which is applied in the field of drug synthesis, can solve the problems of uncontrolled impurities, no public reports of impurities, etc., and achieve the effect of quality assurance, fewer steps, and simple operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
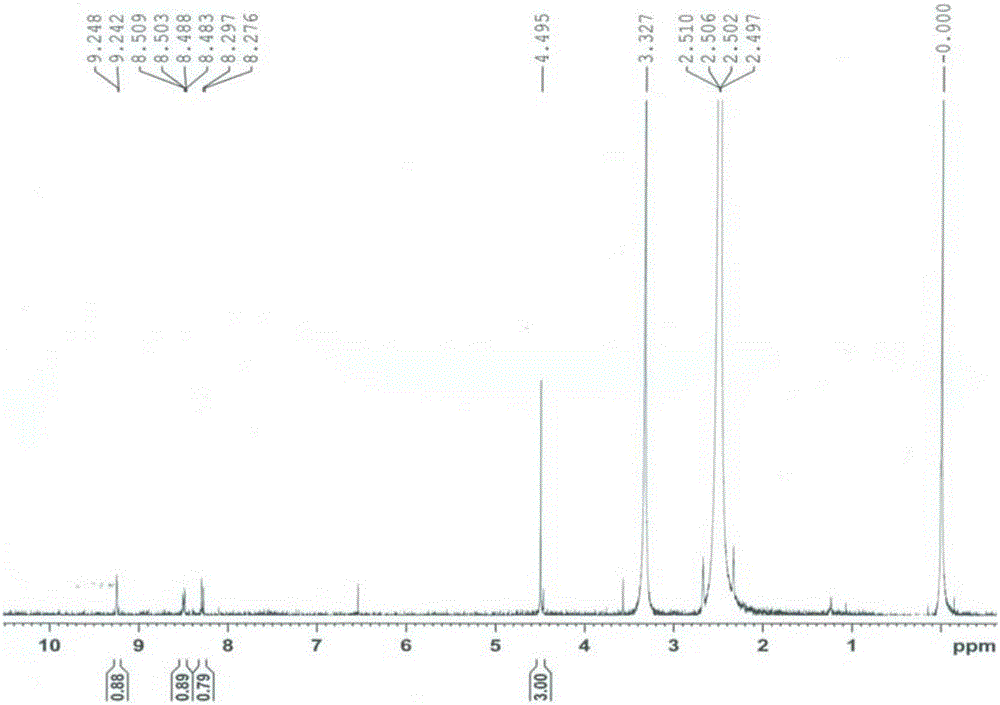
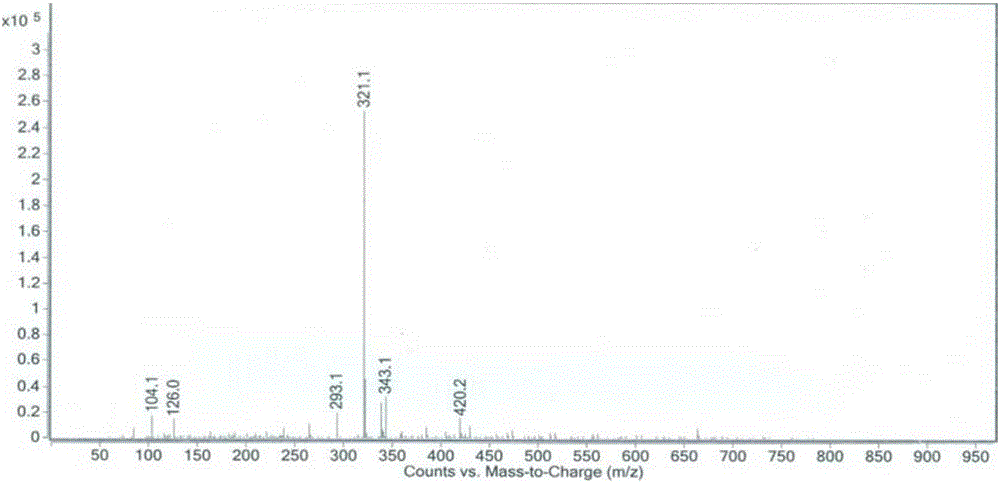
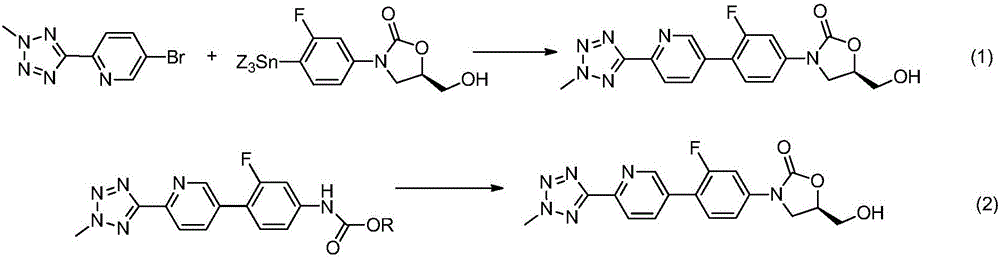
[0030] Nitrogen protection, add 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (10g, 41.6mmol), (6-(2-methyl-2H-tetrazole -5-yl)pyridin-3-yl)boronic acid (8.5g, 41.6mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (304mg, 0.416mmol), potassium carbonate (11.5g, 83.2mmol) and 20mL of a mixture of tetrahydrofuran and water (1:1, V / V), heated to 30 ° C, stirred for 4 hours, TLC detected that the reaction was complete, added 20ml of water, filtered to obtain tedizolid impurity I 12.6g.
Embodiment 2
[0032] Nitrogen protection, add 5-bromo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (21g, 62.4mmol), (6-(2-methyl-2H-tetrazole -5-yl)pyridin-3-yl)boronic acid pinanol ester (11.9g, 41.6mmol), bis(triphenylphosphine)palladium dichloride (292mg, 0.416mmol), sodium tert-butoxide (6.2g, 83.2mmol) and 30mL toluene, the temperature was raised to 110° C., and the reaction was stirred for 5 hours. TLC detected that the reaction was complete, and filtered to obtain 11.9 g of tedizolid impurity I.
Embodiment 3
[0034] Under nitrogen protection, add 5-iodo-2-(2-methyl-2H-tetrazol-5-yl)pyridine (11.9g, 41.6mmol), (6-(2-methyl-2H-tetra Azol-5-yl)pyridin-3-yl)boronic acid (4.25g, 20.8mmol), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (304mg, 0.416mmol), tert Sodium butoxide (8.0 g, 83.2 mmol) and 20 mL of dioxane were heated up to 80° C., stirred and reacted for 2 hours. TLC detected that the reaction was complete, and filtered to obtain 12 g of tedizolid impurity I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com