Tapentadol slow-release pharmaceutical composition and preparation method thereof
A tapentadol and composition technology, applied in the field of tapentadol sustained-release pharmaceutical composition and preparation thereof, can solve the problems of complicated preparation process of sustained-release pellets and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Dissolve ethyl cellulose in ethanol solution, add tapentadol hydrochloride, hydroxypropylmethyl cellulose, and microcrystalline cellulose into the mixing tank according to the ratio in Table 1, and adopt a high-speed shear wet granulation process to Add the ethanol solution of ethyl cellulose to the above-mentioned mixture for granulation, then dry the dry granules (moisture is less than 2%) for dry granulation, add the prescribed amount of magnesium stearate, and use a rotary blender to mix, The resulting blended granules are then compressed into tablets.
[0064] Table 1
[0065]
[0066]
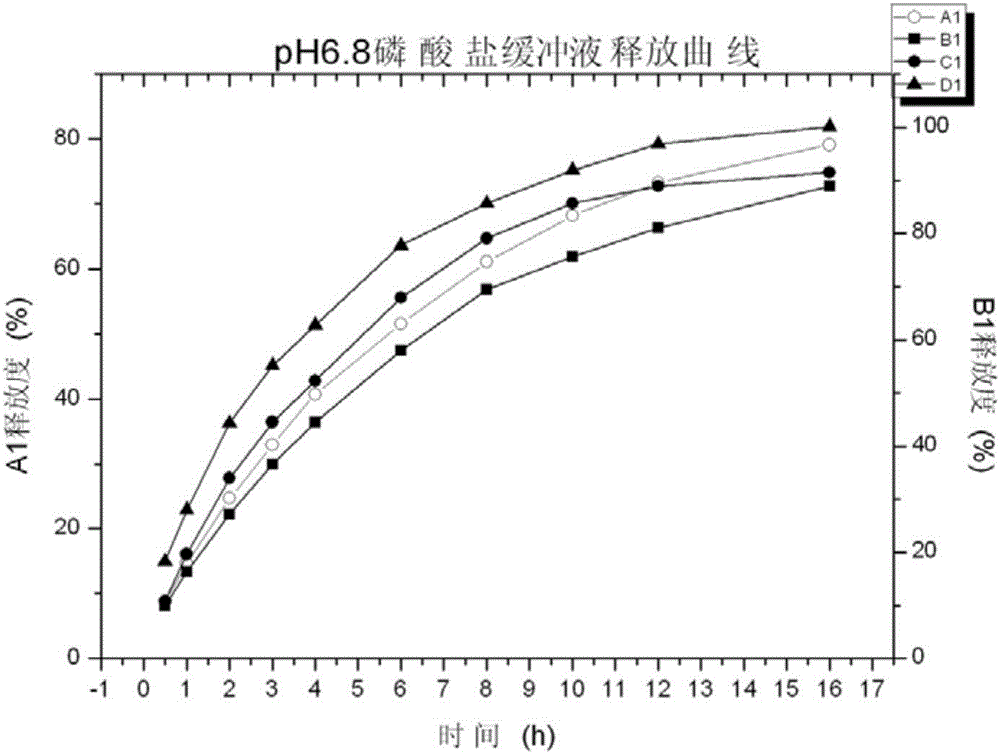
[0067] Investigate the release rate of tapentadol hydrochloride sustained-release tablets in pH6.8 phosphate buffer saline respectively in embodiment 1, adopt the second method paddle method of Chinese Pharmacopoeia dissolution method, adopt 900mL pH6.8 phosphate buffer saline as The dissolution medium was used to carry out the dissolution test of the preparation. The dissol...
Embodiment 2
[0070] Tapentadol hydrochloride, hydroxypropylmethylcellulose, and microcrystalline cellulose were used in the proportions in Table 2, using a fluidized bed granulation process, and after granulation with purified water, the 95% solution of ethyl cellulose Spray on the powder mixture, then dry and granulate, add the prescribed amount of magnesium stearate, mix with a rotary blender, and then compress the obtained blended granules into tablets.
[0071] Table 2
[0072]
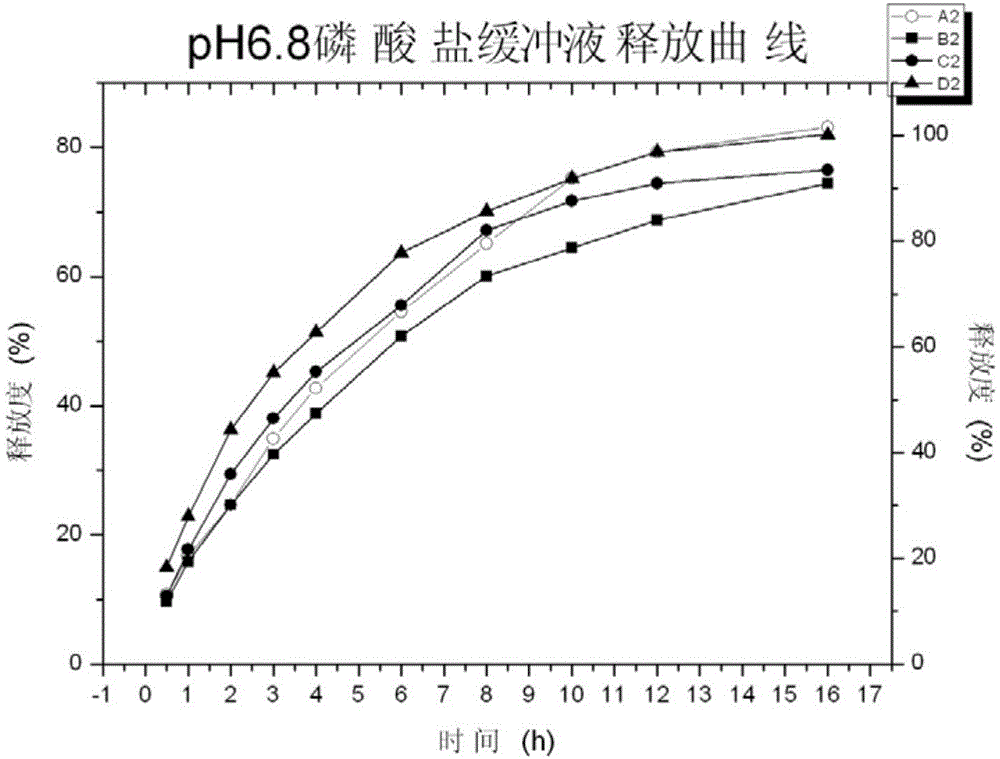
[0073] Investigate the release rate of tapentadol hydrochloride sustained-release tablets in pH6.8 phosphate buffer saline respectively in embodiment 2, adopt the second method paddle method of Chinese Pharmacopoeia dissolution method, adopt 900mL pH6.8 phosphate buffer saline as The dissolution medium was used to carry out the dissolution test of the preparation. The dissolution temperature was 37°C, and the paddle speed was 50rpm. Samples were collected at 0.5, 1, 2, 3, 4, 6, 8, 10, 12, and 16h, and the u...
Embodiment 3
[0078] Dissolve ethyl cellulose in ethanol solution, add tapentadol hydrochloride, hydroxypropyl methylcellulose, and microcrystalline cellulose into the mixing tank according to the ratio in Table 4, and adopt a high-speed shear wet granulation process to Add the ethanol solution of ethyl cellulose to the above-mentioned mixture for granulation, then dry the dry granules (moisture is less than 2%) for dry granulation, add the prescribed amount of magnesium stearate, and use a rotary blender to mix, The resulting blended granules are then compressed into tablets.
[0079] Table 4
[0080]
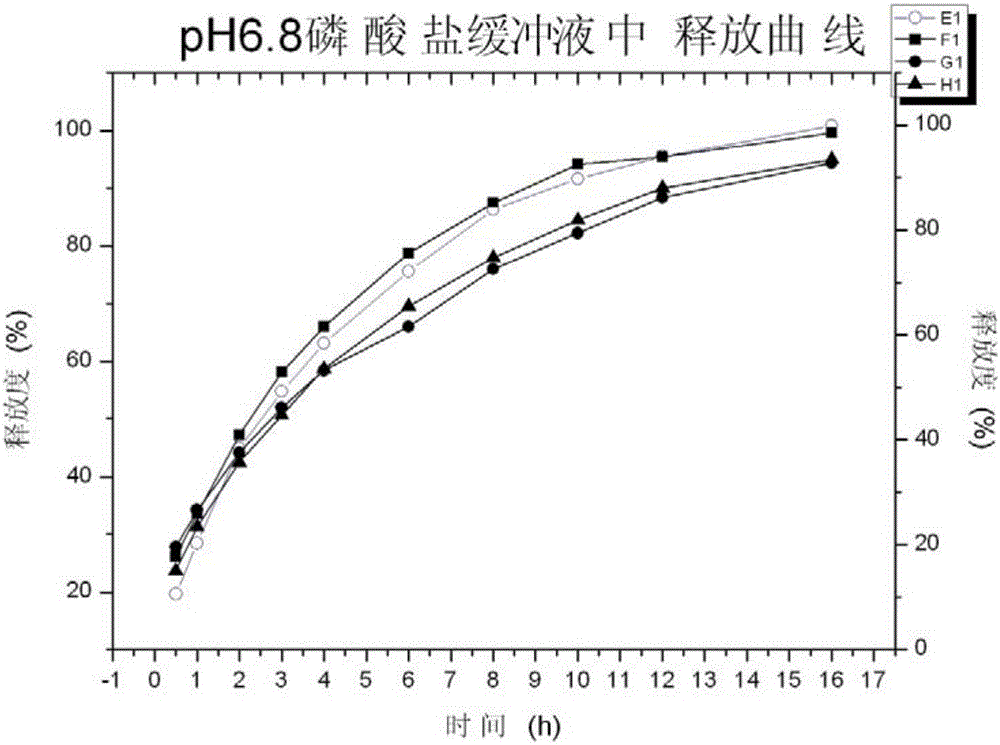
[0081] Investigate respectively the release rate of tapentadol hydrochloride sustained-release tablet in pH6.8 phosphate buffer saline in embodiment 3, adopt the second method paddle method of Chinese Pharmacopoeia dissolution method, adopt 900mL pH6.8 phosphate buffer saline as The dissolution medium was used to carry out the dissolution test of the preparation. The dissolution tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com