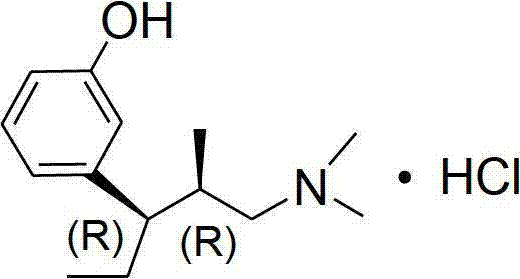
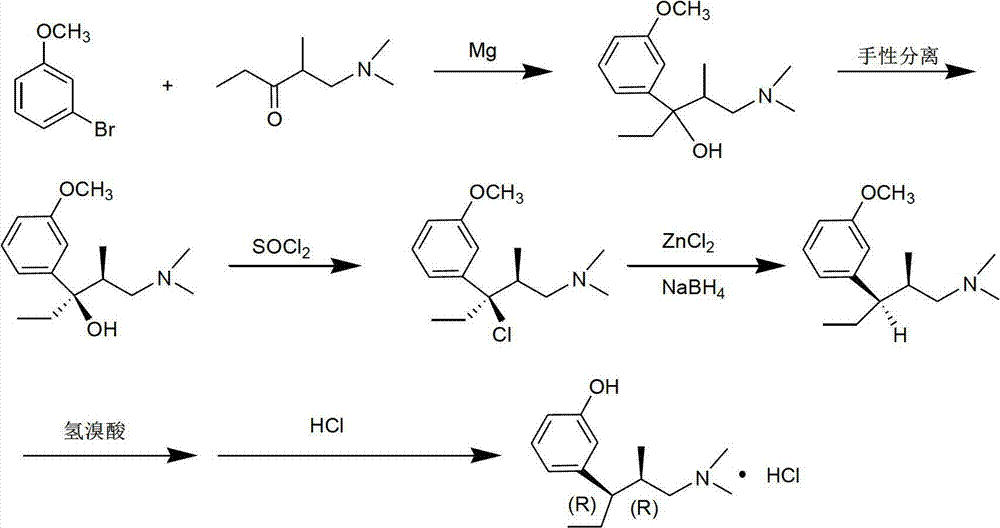
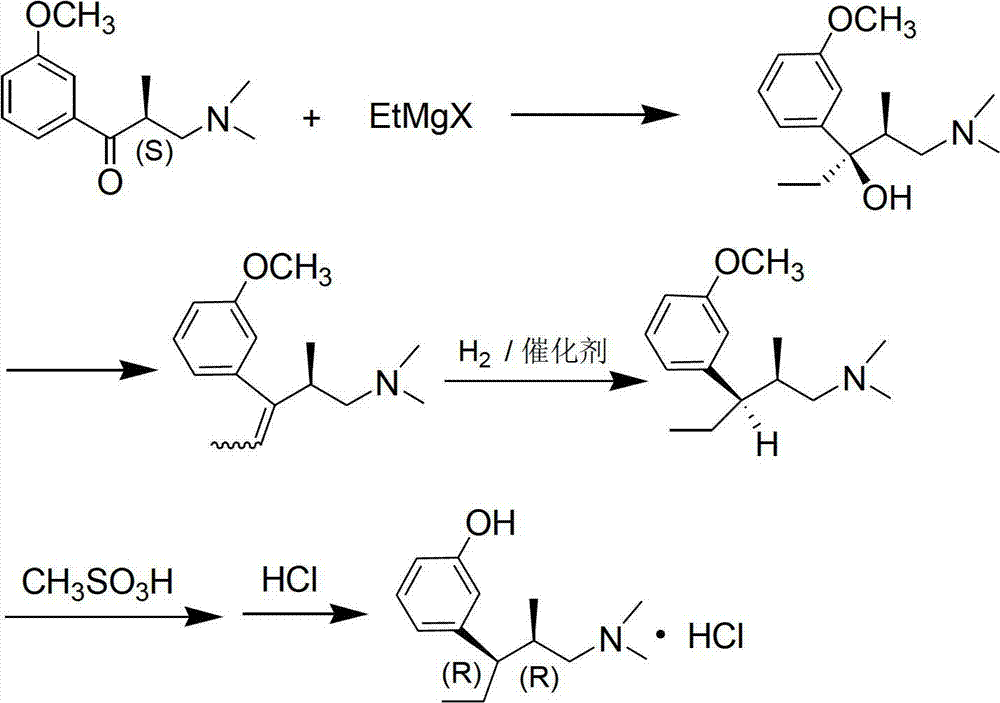
Preparation method of tapentadol hydrochloride and compounds for preparation of tapentadol hydrochloride
A tapentadol and compound technology, applied in the field of drug synthesis, can solve the problems of shortened route, cumbersome operation, and easily corroded equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of 3-allyloxypropiophenone (preparation of formula I compound)
[0088]
[0089] Add the compound 3-hydroxypropiophenone (300.1g), potassium carbonate (450.0g), sodium iodide (40.0g) into 2L of acetone, add 3-chloropropene (208.0g) dropwise under stirring at room temperature, and the addition is complete Afterwards, the reaction mixture was warmed up to reflux temperature and stirred at reflux for 36h. After the reaction, the reaction mixture was cooled to 25° C., and potassium carbonate was removed by suction filtration. After concentrating the acetone under reduced pressure, 1.2 L of ethyl acetate was added to dissolve the oil and 0.5 L of water was added for washing. After washing with water, the organic phase was dried with magnesium sulfate, and the organic phase was concentrated under reduced pressure to obtain oily 3-allyloxypropiophenone (370.8 g), with a yield of 97.6%. 1 HNMR (400MHz, DMSO-d 6 )δ: 7.52 (d, J=8.0Hz, 1H, ArH), 7.49 (s, 1H, ArH), 7...
Embodiment 2
[0091] The preparation of the synthetic formula I compound of 3-allyloxy propiophenone, reaction formula is the same as embodiment 1.
[0092] Add the compound 3-hydroxypropiophenone (30.1g), sodium carbonate (51.0g), sodium bromide (3.5g) into 150ml ethanol, add 3-chloropropene (18.0g) dropwise under stirring at room temperature, and the addition is complete After post-stirring for 1 h, the reaction mixture was warmed to 40 °C and stirred at reflux for 30 h. After the reaction was finished, the reaction mixture was cooled to 25° C., and sodium carbonate was removed by suction filtration. After concentrating the ethanol under reduced pressure, 120 ml of ethyl acetate was added to dissolve the oil and 50 ml of water was added for washing. After washing with water, the organic phase was dried with magnesium sulfate, and the organic phase was concentrated under reduced pressure to obtain oily 3-allyloxypropiophenone (34.8 g), with a yield of 91.3%.
[0093] 1 HNMR (400MHz, DMSO...
Embodiment 3
[0095] The preparation of the synthetic formula I compound of 3-allyloxy propiophenone, reaction formula is the same as embodiment 1.
[0096] The compound 3-hydroxypropiophenone (25.3g), sodium bicarbonate (38.0g), potassium bromide (2.5g) was added to 100ml of toluene, and 3-chloropropene (18.0g) was added dropwise under stirring at room temperature. After stirring for 1 h after completion, the reaction mixture was warmed up to 110° C. and stirred at reflux for 28 h. After the reaction was finished, the reaction mixture was cooled to 25° C., and sodium bicarbonate was removed by suction filtration. After concentrating the toluene under reduced pressure, 120 ml of ethyl acetate was added to dissolve the oil and 50 ml of water was added for washing. After washing with water, the organic phase was dried with magnesium sulfate, and the organic phase was concentrated under reduced pressure to obtain oily 3-allyloxypropiophenone (24.8 g), with a yield of 77.4%.
[0097] 1 HNMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com