Tapentadol hydrochloride paracetamol orally disintegrating tablet and preparation method thereof
A technology of paracetamol and tapentadol hydrochloride is applied in the field of compound oral disintegrating tablets containing tapentadol hydrochloride and paracetamol hydrochloride and the field of preparation thereof, which can solve difficulty in swallowing, cannot fully meet the needs of patients for medication, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 37.5mg / 325mg Tapentadol Hydrochloride Orally Disintegrating Acetaminophen Tablets
[0040] 1. Prescription
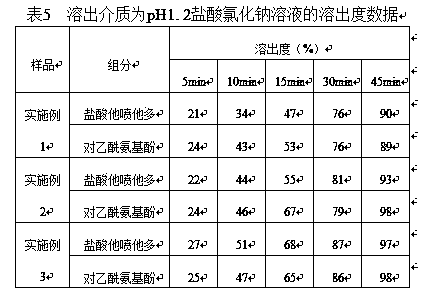
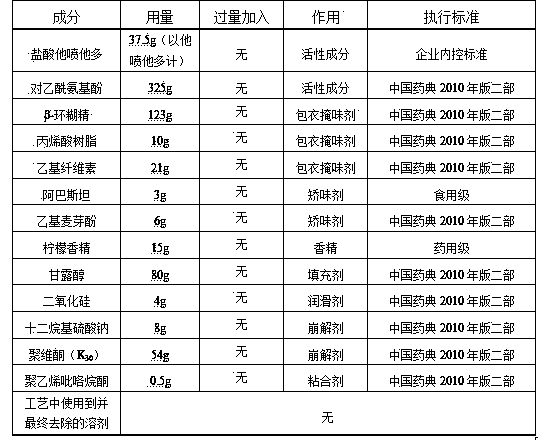
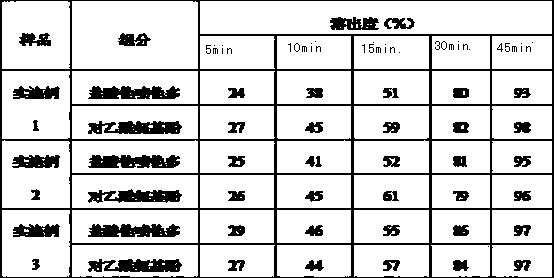
[0041] Table 1 Prescription Composition of 37.5mg / 325mg Tapentadol Hydrochloride Orally Disintegrating Acetaminophen Tablets (1000 Tablets)
[0042]
[0043] 2. Preparation process
[0044] (1) Mix 3g of the flavoring agent aspartame and 80g of the filler mannitol, add 0.5g of the binder polyvinylpyrrolidone, pass through a 20-mesh sieve to prepare wet granules, and dry at 60-65°C to obtain granules ( I);
[0045] Said binder is polyvinylpyrrolidone;
[0046] (2) Mix 37.5g tapentadol hydrochloride (calculated as tapentadol) with 380g saturated ethanol solution of coating taste-masking agent β-cyclodextrin, mix tapentadol hydrochloride and β-cyclodextrin Add to the ethanol solution respectively, stir at high speed with a high-speed mixer for 3 to 5 hours, then spread the stirred solution on a glass dish, put it in a 55°C oven for drying, take i...
Embodiment 2
[0051] Example 2 56.25mg / 325mg Tapentadol Hydrochloride Acetaminophen Orally Disintegrating Tablets
[0052] 1. Prescription
[0053] Table 2 Prescription Composition of 56.25mg / 325mg Tapentadol Hydrochloride Orally Disintegrating Acetaminophen Tablets (1000 Tablets)
[0054]
[0055] 2. Preparation process
[0056] (1) Mix 4g of flavoring agent aspartame and 95g of filler mannitol, add 0.5g of binder polyvinylpyrrolidone, pass through a 20-mesh sieve to make wet granules, and dry at 60-65°C to obtain granules ( I);
[0057] Said binder is polyvinylpyrrolidone;
[0058] (2) Mix 56.25g of tapentadol hydrochloride (calculated as tapentadol) with 570g of saturated ethanol solution of coating taste-masking agent β-cyclodextrin, mix tapentadol hydrochloride and β-cyclodextrin Add to the ethanol solution respectively, stir at high speed with a high-speed mixer for 3 to 5 hours, then spread the stirred solution on a glass dish, put it in a 55°C oven for drying, take it out...
Embodiment 3
[0063] Example 3 75mg / 325mg Tapentadol Hydrochloride Orally Disintegrating Acetaminophen Tablets
[0064] 1. Prescription
[0065] Table 3 Prescription Composition of 75mg / 325mg Tapentadol Hydrochloride Orally Disintegrating Acetaminophen Tablets (1000 Tablets)
[0066]
[0067] 2. Preparation process
[0068] (1) Mix 4g of flavoring agent aspartame and 110g of filler mannitol, add 0.5g of binder polyvinylpyrrolidone, pass through a 20-mesh sieve to prepare wet granules, and dry at 60-65°C to obtain granules ( I);
[0069] Said binder is polyvinylpyrrolidone;
[0070] (2) Mix 37.5g tapentadol hydrochloride (calculated as tapentadol) with 760g saturated ethanol solution of coating taste-masking agent β-cyclodextrin, mix tapentadol hydrochloride and β-cyclodextrin Add to the ethanol solution respectively, stir at high speed with a high-speed mixer for 3 to 5 hours, then spread the stirred solution on a glass dish, put it in a 55°C oven for drying, take it out, and crush...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com