Novel thiazole derivatives, and preparation method and application thereof
A technology of thiazoles and derivatives, applied in the field of medicinal chemistry, can solve the problems of many adverse reactions, unsatisfactory overall therapeutic effect, and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
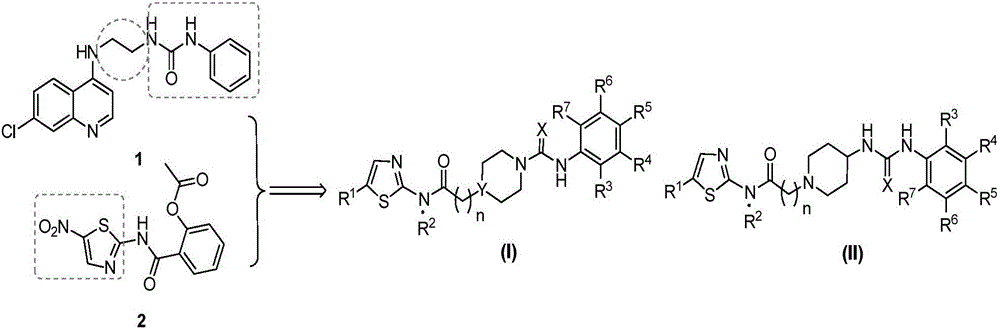
[0119] Example 1: 4-(2-((5-nitrothiazol-2-yl)amino)-2-oxoethyl)-N-phenylpiperazine-1-carboxamide (compound I-1, code XQH-3-13) preparation method
[0120] Preparation of intermediate 2-chloro-N-(5-nitrothiazol-2-yl) (1-1)
[0121] Dissolve 5-nitro2-aminothiazole (1eq) and triethylamine (1.2eq) in dichloromethane, add chloroacetyl chloride (1.2eq) dropwise at 0°C, and continue the reaction from the temperature rise after the addition After 5 h, the reaction was detected by TLC, and the reaction was stopped because there was no raw material remaining. Add 100ml of distilled water to the reaction solution, the aqueous phase was extracted three times with dichloromethane (3×100ml), the combined organic layer was washed twice with saturated sodium chloride solution (2×100ml), dried over anhydrous magnesium sulfate, and then evaporated Concentrated crude. The crude product was purified and separated by silica gel column (dichloromethane:methanol=150:1) to obtain the target compo...
Embodiment 2
[0129] Embodiment 2: N-(4-fluorophenyl)-4-(2-((5-nitrothiazol-2-yl)amino)-2-oxoethyl)piperazine-1-carboxamide (I -2, the code name is the preparation method of XQH-2-78)
[0130] The preparation method is the same as I-1, and the yield is 72%. 1 H NMR (400MHz, DMSO-d6 ): δ8.63(s,1H),8.57(s,1H),7.48-.42(m,2H),7.07(t,J=8.9Hz,2H),3.53(s,2H),3.52-3.48 (m,5H),2.67-2.62(m,4H)ppm; ESI-MS: 408.1[M-H] - .
Embodiment 3
[0131] Embodiment 3: N-(4-chlorophenyl)-4-(2-((5-nitrothiazol-2-yl)amino)-2-oxoethyl)piperazine-1-carboxamide (I -3, code name is the preparation method of XQH-2-80)
[0132] The preparation method is the same as I-1, and the productive rate is 70%, 1 H NMR (400MHz, DMSO-d 6 ): δ8.67(s,1H),8.63(s,1H),7.49(d,J=8.6Hz,2H),7.28(d,J=8.6Hz,2H),3.52(m,6H),2.64 (s,4H).ESI-MS: 423.0[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com