Method for culturing and cryopreservation of CIK cells by applying serum-free lymphocyte culture medium and obtained CIK cells
A lymphocyte and cell culture technology, applied in the field of cell biology, can solve problems affecting culture, cell death, and low cell viability, and achieve the effect of improving cell activity and ensuring amplification efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
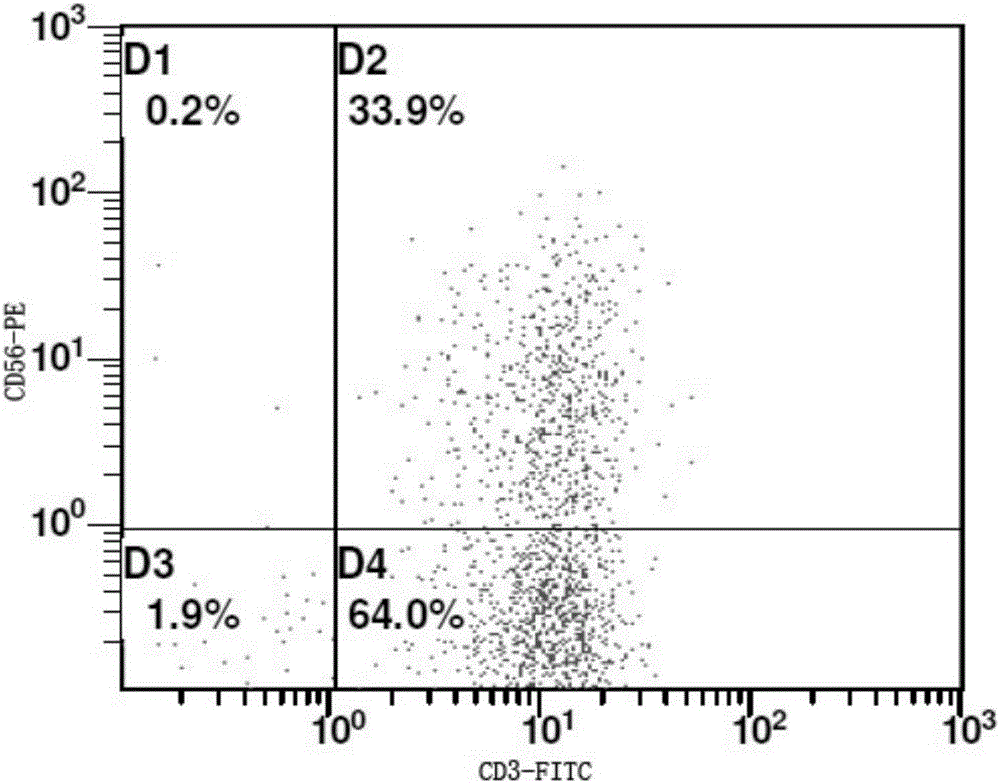
Image
Examples
Embodiment 1
[0067] (1) Coat a T175 culture flask with 10ml of serum-free lymphocyte medium containing Retronectin (1ug / ml) and CD3mAb (5ug / ml) protein, CO 2 Incubate for 2 hours.
[0068] (2) About 60 ml of conventional umbilical cord blood was collected, centrifuged by Ficoll-Hypaque density gradient, and centrifuged at 2000 rpm / min for 25 min (the acceleration was adjusted to 1 and the brake was adjusted to 1 in the centrifugation parameters).
[0069] (3) Collect and number the upper and middle plasma in step (2) in a 50ml centrifuge tube, and place the collected plasma in a 56°C water bath for 30min to inactivate complement. After that, centrifuge at 3000rpm / min for 6min to remove the precipitates in the plasma, and transfer 27ml of the upper serum to a new centrifuge tube, and place it in a 4°C refrigerator for later use.
[0070] (4) Collect the buffy coat cells in step (2), wash twice, and finally obtain 4.6*10 7 a monocyte.
[0071] (5) Take the coated T175 culture flask. Acco...
Embodiment 2
[0081] (1) Coat a T175 culture flask with 10ml of serum-free lymphocyte medium containing Retronectin (1ug / ml) and CD3mAb (5ug / ml) protein, overnight at 4°C.
[0082] (2) About 60 ml of collected conventional peripheral blood was collected, centrifuged with Ficoll-Hypaque density gradient, and centrifuged at 2000 rpm / min for 25 min (the acceleration was adjusted to 1 and the brake was adjusted to 1 in the centrifugation parameters).
[0083] (3) Collect and number the upper and middle plasma in step (2) in a 50ml centrifuge tube, and place the collected plasma in a 56°C water bath for 30min to inactivate complement. After that, centrifuge at 3000rpm / min for 6min to remove the precipitates in the plasma, and transfer 33ml of the upper serum to a new centrifuge tube, and place it in a 4°C refrigerator for later use.
[0084] (4) Collect the buffy coat cells in step (2), wash twice, and finally obtain 8*10 7 a monocyte.
[0085] (5) Take the coated T175 culture flask. Accordin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com