Preparation of single chain antibody of human liver cancer marker and application thereof
A single-chain antibody and marker technology, applied in the fields of application, botany equipment and methods, biochemical equipment and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
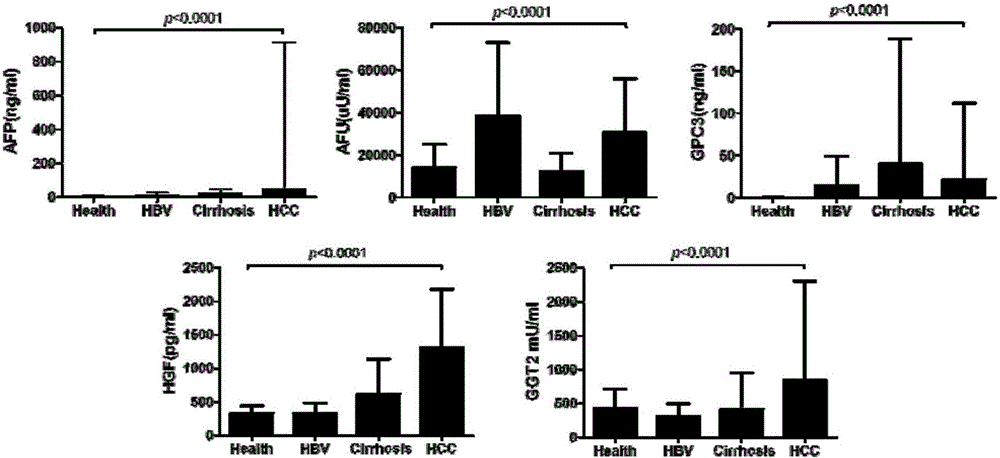
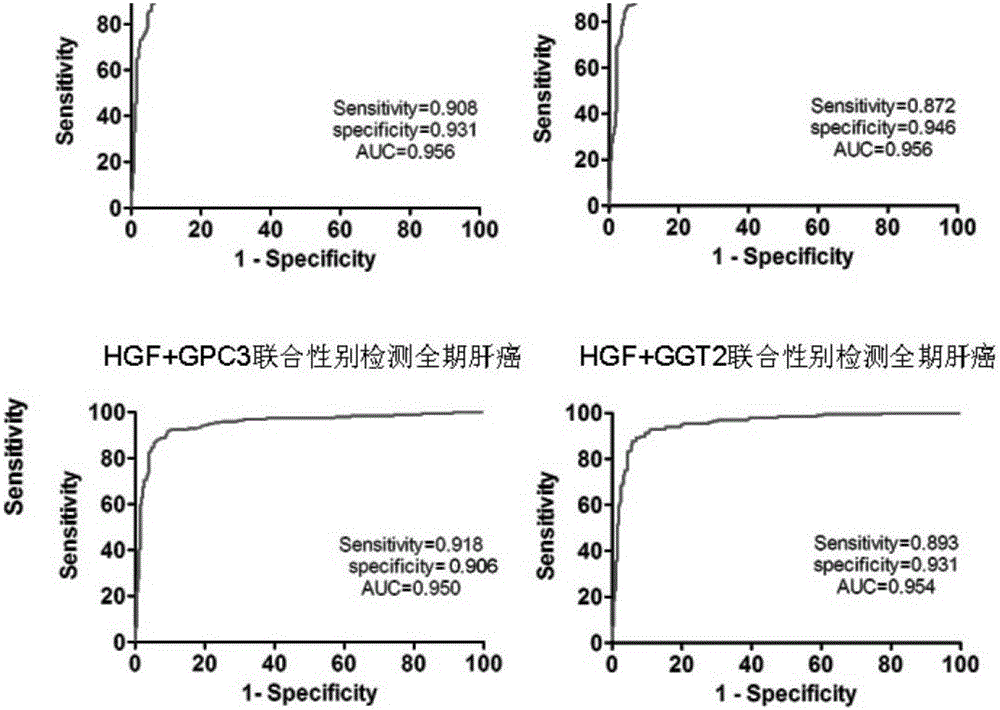
[0169] Example 1: Preparation of HGF antibody animal immunization and immune titer determination (taking HGF as an example, the rest of the antibody steps are the same or similar)
[0170] 1) Three 7-week-old female Balb / c mice were first immunized with 100 μg of HGF fusion protein (optionally such as HGF protein with a tagged protein such as His) per mouse, plus an equal volume of Freund's complete adjuvant, Emulsify and mix to form a water-in-oil chylus through a three-way connection, and inject it subcutaneously at multiple points.
[0171] 2) After an interval of three weeks, the dose of antigen was halved, mixed with Freund's incomplete adjuvant and normal saline, and injected intraperitoneally. After four times of immunization in the same way, venous blood was taken to measure the titer.
[0172] 3) Coating HGF protein, 4ng / ml. overnight at 4°C;
[0173] 4) After washing once with PBS, block with 0.2% BSA at room temperature for 1 hour;
[0174] 5) Serum was diluted ...
Embodiment 2
[0178] Example 2: Cell Fusion and Screening of Fusion Cells
[0179] 1) Two 7-week-old Balb / c mice were killed, and macrophages were taken as feeder cells and spread in six 96-well plates;
[0180] 2) The mice three days after intravenous injection were sacrificed, and spleens were taken, fused with myeloma cells SP2 / 0 and plated into six 96-well plates by infinite dilution. The culture condition is DMEM / F12 culture medium containing 20% FBS and 1×HAT;
[0181] 3) Observing the growth of clones after 10 days, supplementing 1×HAT-containing DMEM / F12 culture medium twice during this period;
[0182] 4) Observing the hybridoma cells under a microscope, the round and bright grape clusters can start to detect the specificity. Use the ELISA method to screen the positive wells that can specifically bind to the antigen for subcloning;
[0183] 5) Subcloning by limiting dilution method: the giant cells were taken as cultured cells and spread in six 96-well plates. Adjust the numb...
Embodiment 3
[0186] Example 3: Preparation and purification of ascites
[0187] 1) Prepare 5 female Balb / c mice about 10 weeks old, intraperitoneally inject 500μl pristine / mouse, and after 7-21 days, each mouse intraperitoneally injects 2×10 6 A hybridoma cell in the logarithmic growth phase was observed closely after 10 days, and the ascites was taken out when the mice were killed as much as possible. 3000rpm, 15min centrifugation to remove impurities and grease.
[0188] 2) Precipitation of immunoglobulin by salting-out method: Dilute the ascites water by one time with PBS, and add saturated ammonium sulfate to make the final concentration 33%. Shake while adding dropwise. Place at 4°C for more than 4 hours. Centrifuge at 12000rpm for 15min and discard the supernatant. The precipitate was dissolved with an appropriate amount of PBS, put into a dialysis bag, and dialyzed overnight at 4°C.
[0189] 3) Take out the dialysate, use the swimming pump to put the liquid on the column, the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com