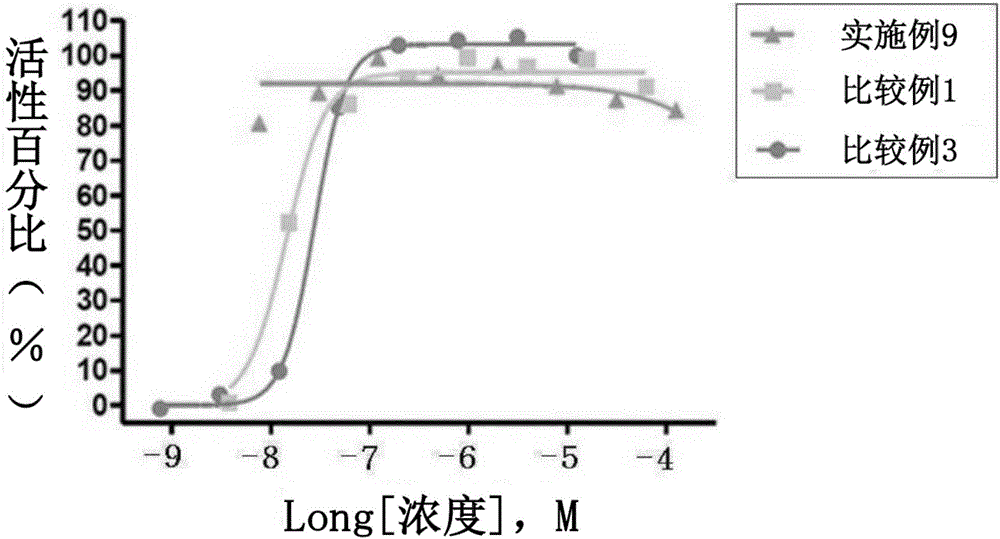
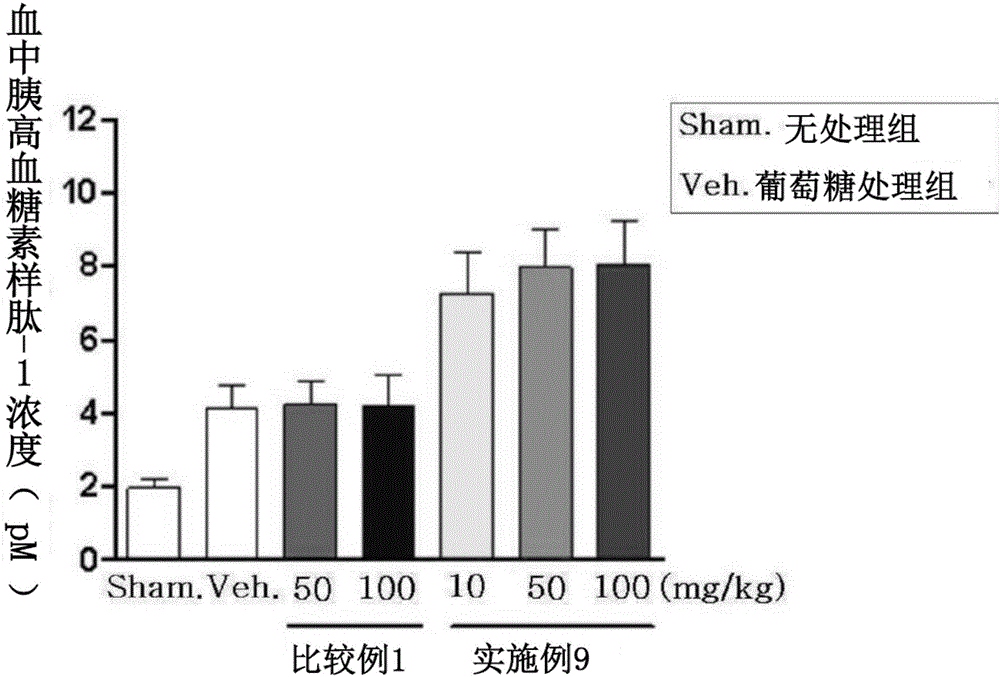
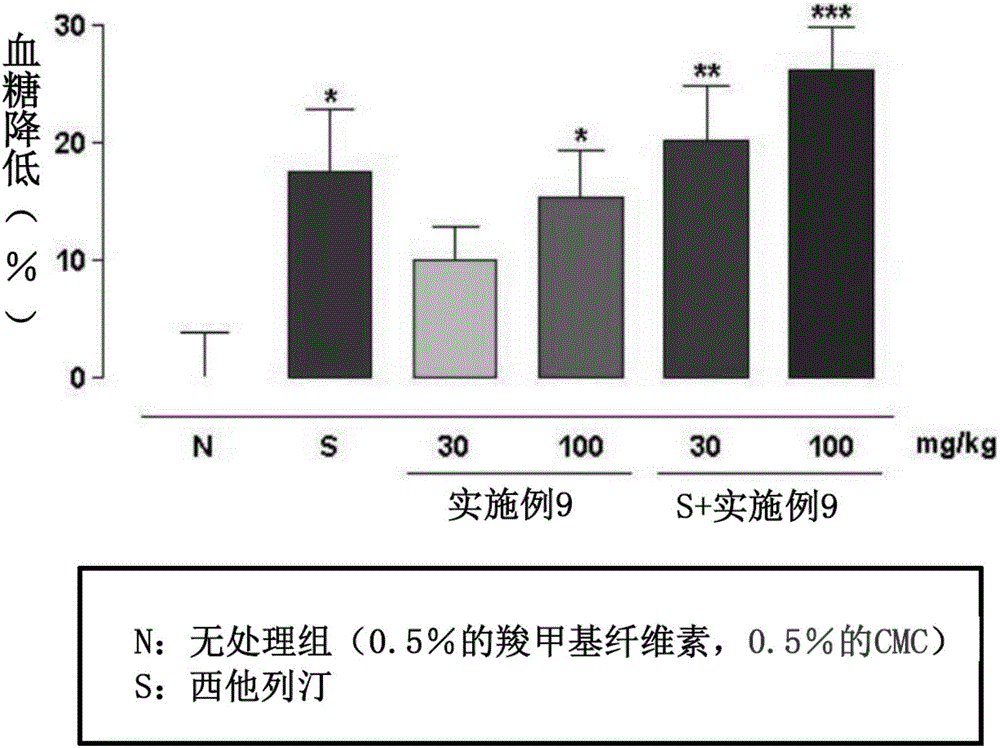
Composite preparation, containing novel 3-(4-(benzyloxy)phenyl)hex-4-inoic acid derivative and another active ingredient, for preventing or treating metabolic diseases
A metabolic disease, benzyloxy technology, applied in the field of pharmaceutical compositions for the prevention or treatment of metabolic diseases, can solve problems such as low therapeutic responsiveness, and achieve excellent blood sugar lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] The preparation method of the compound represented by Chemical Formula 1 of the present invention is as follows.
[0120] Preparation method 1
[0121] As shown in the following reaction formula 1, the preparation method of the compound represented by chemical formula 1 of the present invention comprises: step 1, making the compound represented by chemical formula 2 and the compound represented by chemical formula 3 undergo condensation reaction to prepare the compound represented by chemical formula 4 a compound; and step 2 of performing a reduction reaction on the compound represented by Chemical Formula 4 prepared in the above Step 1 to prepare the compound represented by Chemical Formula 1.
[0122] Reaction 1:
[0123] (In the above reaction formula 1, R 1 , R 2 , R 3 , R 4A , R 4B , R 5 , A, E, n and X are the same as defined in the above chemical formula 1; Y is C 1-10 straight-chain or side-chain alkyl).
[0124] Hereinafter, the preparation method of...
preparation example 1
[0203] Preparation of 3-(4-hydroxyphenyl)-4-hexenoic acid ethyl ester
[0204]
[0205] Under a nitrogen atmosphere, inject 3-(4-hydroxyphenyl)-hex-4-enoic acid (20.0 g) and ethanol (200 mL) into a 250 mL flask, and after stirring to complete the dissolution, slowly Sulfuric acid (9.6 mL) was instilled dropwise. Then, reflux stirring was performed for 6 hours or more, and when the reaction was complete, distilled water (150 mL) was dripped slowly, and ethyl acetate (200 mL) was inject|poured, and extraction was performed. The extracted layers were dried under reduced pressure to obtain the target compound (19.5 g, 85.7%).
[0206] 1 H NMR (400MHz, CDCl 3 ): δ7.25(2H, d), 6.78(2H, d), 4.95(1H, s), 4.14(2H, m), 4.04(1H, m), 2.68(2H, m), 1.84(3H, d), 1.29 (3H,t).
preparation example 2
[0207] Preparation of (S)-3-(4-hydroxyphenyl)-4-hexenoic acid ethyl ester
[0208]
[0209] Under a nitrogen atmosphere, (S)-3-(4-hydroxyphenyl)-hex-4-enoic acid (20.0 g) and ethanol (200 mL) were injected into a 250 mL flask, and after dissolution was completed by stirring, Sulfuric acid (9.6 mL) was slowly dripped at room temperature. Then, reflux stirring was performed for 6 hours or more, and when the reaction was completed, distilled water (150 mL) was dripped slowly, and extraction was performed with ethyl acetate (200 mL). The extracted layers were dried under reduced pressure to obtain the target compound (21.2 g, 93.2%).
[0210] 1 H NMR (400MHz, CDCl 3 ): δ7.25(2H, d), 6.78(2H, d), 4.95(1H, s), 4.14(2H, m), 4.04(1H, m), 2.68(2H, m), 1.84(3H, d), 1.29 (3H,t).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com