Application of aromatic ester compounds in preparing anti-tumor drugs
A technology of ester compounds and anti-tumor drugs, applied in the field of anti-tumor drugs, can solve the problems of biological activity evaluation and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
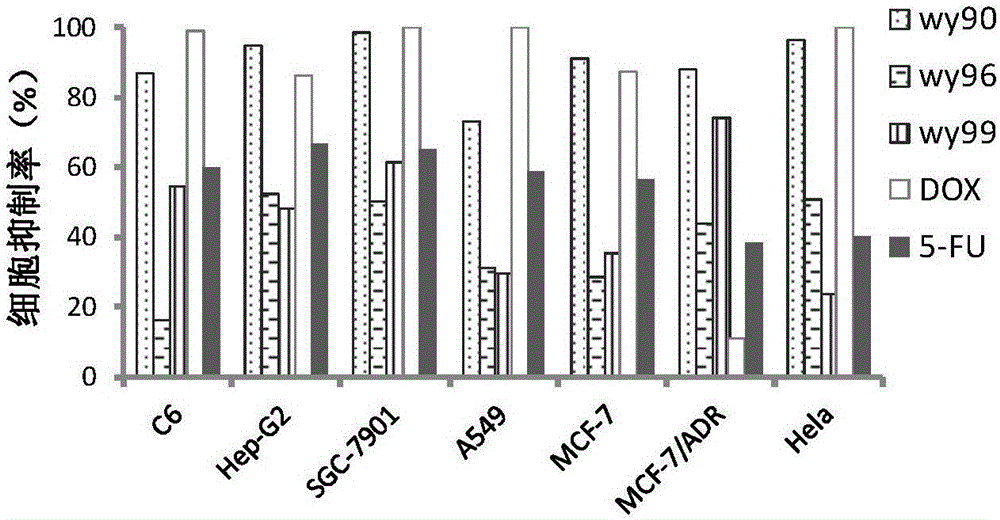
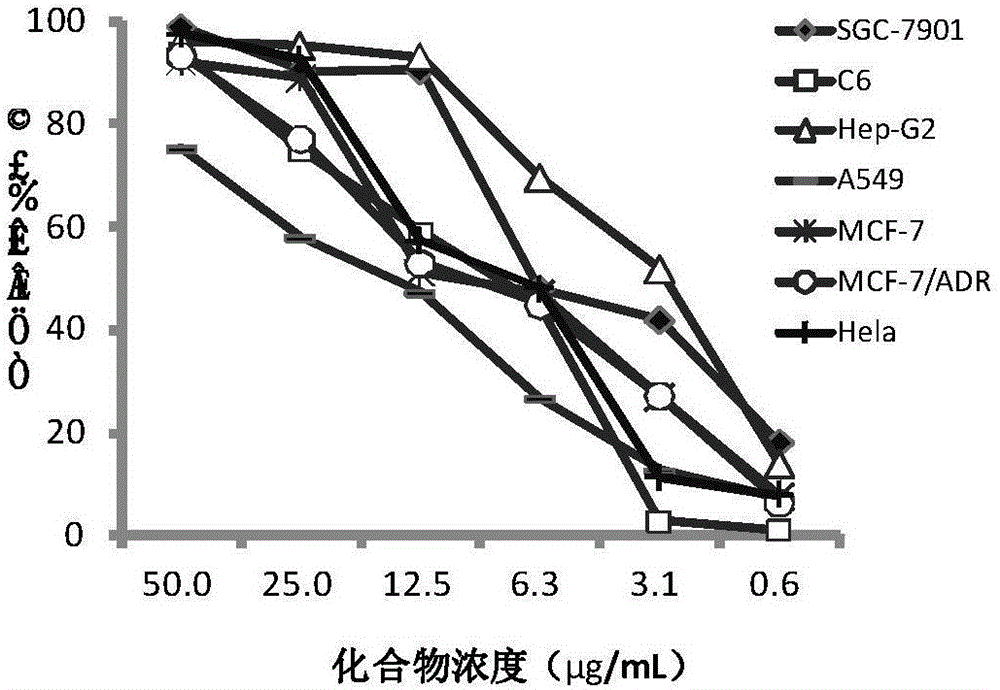
[0023] [Example 1] In vitro anti-tumor activity evaluation of compounds
[0024] Tested tumor cells: human liver cancer cells HepG2, human lung cancer cells A549, human breast cancer cells MCF-7, human breast cancer cells resistant to doxorubicin (MCF-7 / ADR), human gastric cancer cells SGC-7901, human cervical cancer cells Cell Hela, human glioma cell C6.
[0025] Cell culture: GIBCO DMEM medium, 10% fetal bovine serum and 0.01% L-glutamine were prepared as culture medium. Cultured cell lines were placed at 37°C, 5% CO 2 The cells were routinely cultured and subcultured under saturated humidity, and the cells in the logarithmic growth phase were used in the experiments.
[0026] In vitro anti-tumor activity evaluation (MTT method):
[0027] The above tumor cells were plated in 96-well plates respectively, and kept at 37°C, 5% CO 2 After the monolayer was grown in the incubator, the cell culture medium was discarded, and the cell maintenance medium containing different conc...
Embodiment 2
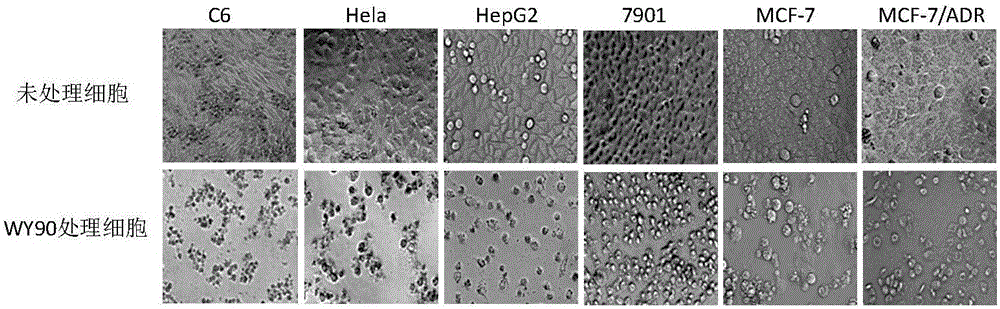
[0036] [Example 2] The tumor cell pathological effect caused by compound WY90
[0037] We further documented the tumor cytopathic effect induced by WY90 by microscopy. The specific implementation method is as follows:
[0038] HepG2, MCF-7, MCF-7 / ADR, SGC-7901, Hela, and C6 tumor cells in the logarithmic growth phase were respectively plated on 24-well plates, cultured in a 5% CO2 incubator at 37°C, and then discarded. Cell-free culture medium was added to the cell maintenance medium containing 20 μg / mL WY90 (Hela cells plus 10 μg / mL) to continue the culture. At 48 hours, the cells were visually observed under a microscope and photographed.
[0039] The tumor cell pathological effect caused by compound WY90 is as follows: image 3 shown. Under the microscope, the untreated tumor cells grew well, with firm adherence, plump shape, and clear borders. 20μg / mL WY90 treatment for 48 hours led to the apoptosis of all tumor cells, and the cells became round in different forms and fe...
Embodiment 3
[0040] [Example 3] Evaluation of tumor cell apoptosis activity caused by compound WY90
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com