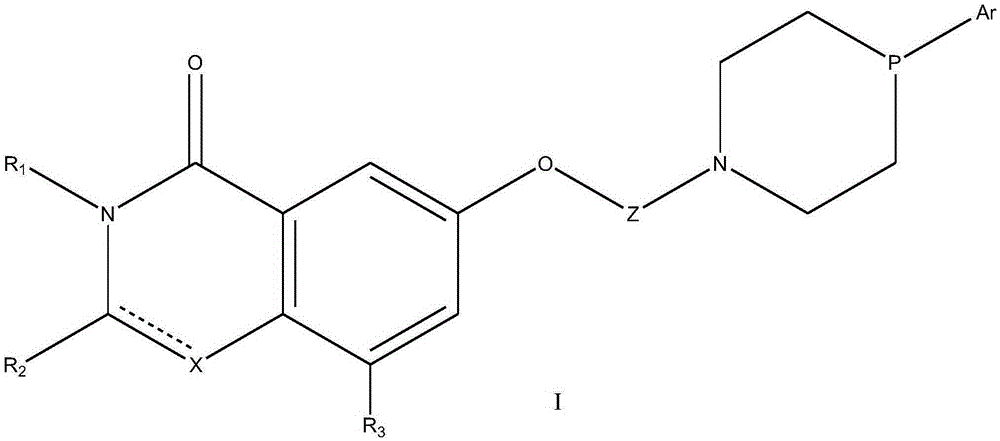
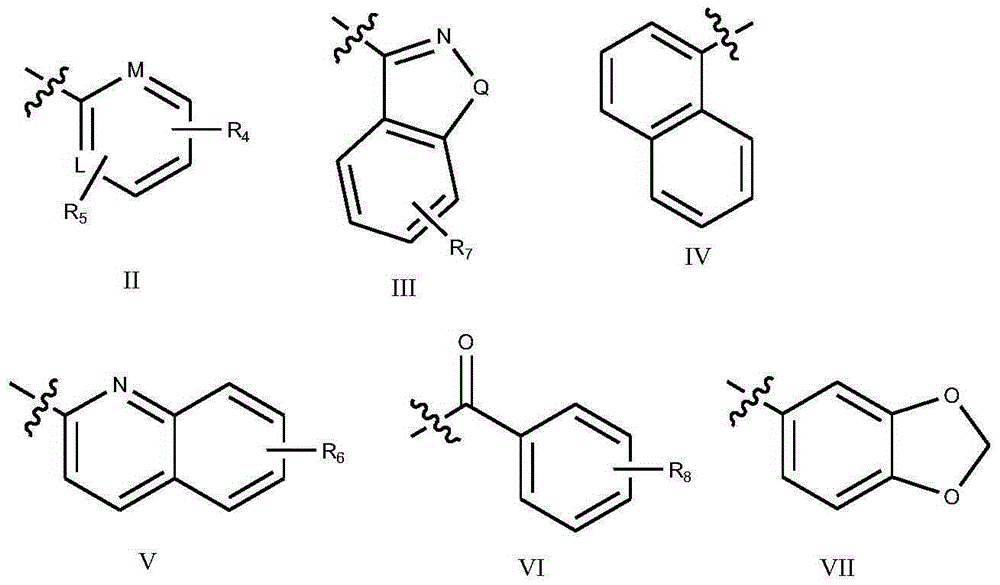
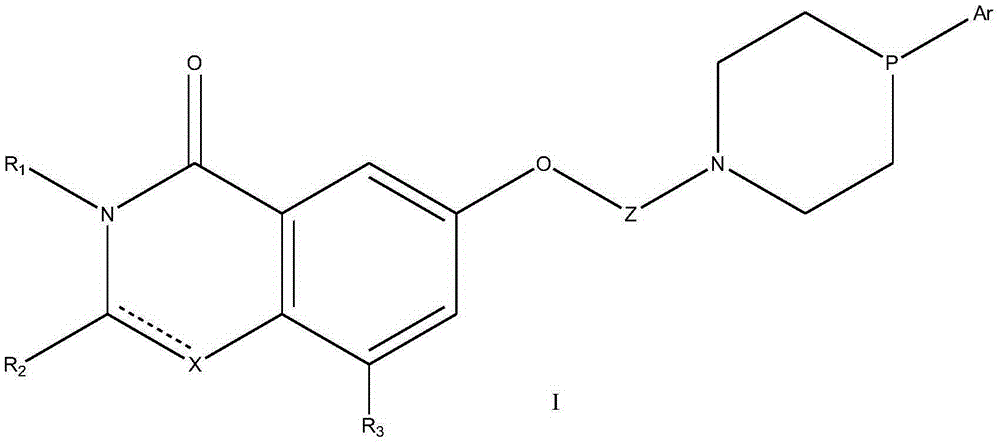
Lactam derivative and application thereof
A compound, halogen technology, applied in the field of medicine, can solve problems such as QT interval prolongation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1, 7-(4-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidinyl-1-yl)butoxy)-2-methyl-3,4 -Dihydroisoquinolin-1(2H)-one (1)
[0095](1) 4-Methoxyphenethylamine (0.1mol) was dissolved in 300ml of dichloromethane, cooled to 0°C in an ice bath, 20ml of triethylamine was added, and ethyl chloroformate (0.15mmol) was slowly added dropwise thereto , After adding, react at room temperature for 12h. After the reaction was completed, the reaction solution was washed with water and 10% dilute hydrochloric acid solution, the organic layer was dried with anhydrous magnesium sulfate, the solvent was evaporated under reduced pressure, and the residue was washed with petroleum ether. After drying, 20.3 g of a yellow oil was obtained, with a yield of 91 %, MS (ESI) m / z 223.3 ([M+H] + ).
[0096] (2) Dissolve 0.2 mol of phosphorus pentoxide in 200 ml of methanesulfonic acid, add 0.1 mol of the yellow oil prepared in the first step into it in batches, react at 140 ° C, and after the rea...
Embodiment 2
[0103] Example 2, 7-(4-(4-(2-methoxyphenyl)piperazin-1-yl)butoxy)-2-methyl-3,4-dihydroisoquinoline-1( 2H)-ketone (2)
[0104] Using 1-(2-methoxyphenyl)piperazine instead of 6-fluoro-3-(piperidinyl-4-yl)benzo[d]isoxazole as raw material, the target compound was prepared according to the method of Example 1 , and the structural formula is shown in number (2) in Table 1. 1 H-NMR (600MHz, CDCl 3 )δ1.72-1.86(m,4H),2.50(t,2H,J=12Hz),2.69-2.70(m,3H),2.94(t,2H,J=12Hz),3.12-3.16(m,2H ),3.18(s,3H),3.54(t,2H,J=12Hz),3.87(s,3H),4.05(t,2H,J=6Hz),6.86-6.88(m,1H),6.91-7.07 (m, 5H), 7.61 (d, 1H, J=6Hz). MS (ESI) m / z 424.3 ([M+H] + ).
Embodiment 3
[0105] Example 3, 2-methyl-7-(4-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)butoxy)-3,4-dihydroisoquinoline -1(2H)-one(3)
[0106] Use 1-(3-(trifluoromethyl)phenyl) instead of 6-fluoro-3-(piperidinyl-4-yl)benzo[d]isoxazole as raw material, and prepare the target according to the method of Example 1 Compound, structural formula as shown in number (3) in Table 1. 1 H-NMR (600MHz, CDCl 3 )δ1.73-1.88(m, 4H), 2.21-2.22(m, 2H), 2.50(t, 2H, J=12Hz), 2.64-2.66(m, 3H), 2.94(t, 2H, J=12Hz ),3.18(s,3H),3.26-3.28(m,3H),3.55(t,2H,J=12Hz),4.06(t,2H,J=6Hz),6.96-6.98(m,1H),7.06 -7.10(m,3H),7.12-7.14(m,1H),7.34-7.36(m,1H),7.61(d,1H,J=6Hz). MS(ESI) m / z 462.2([M+H ] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com