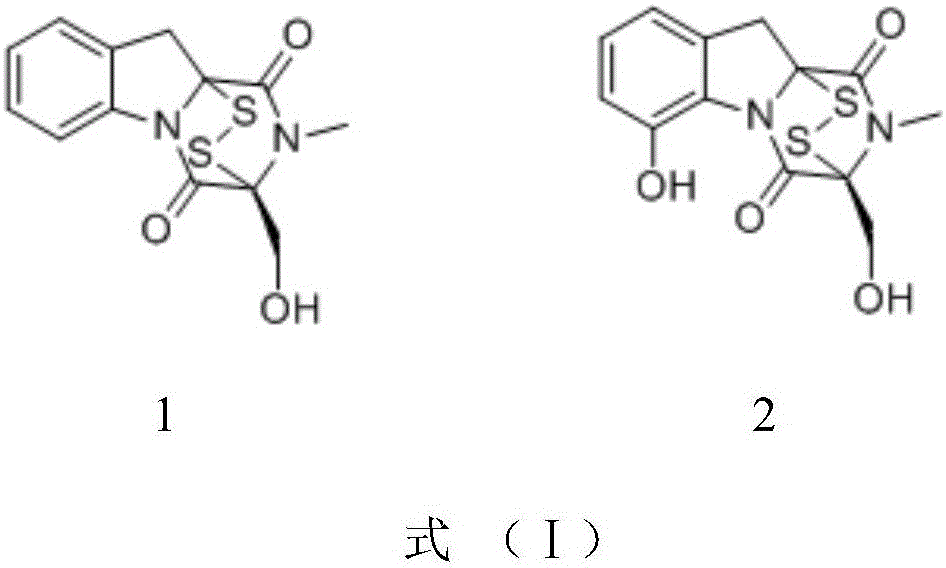
Application of two gliotoxins in preparation of biopesticide
A technology of gliotoxin, biopesticide, applied in the field of agriculture and biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
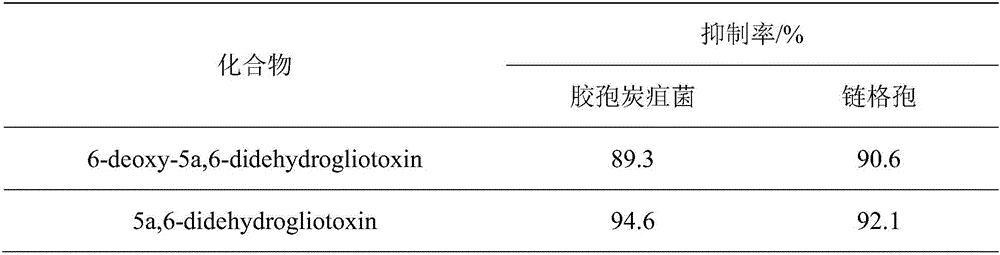
[0020] The inhibitory effects of compounds 6-deoxy-5a,6-didehydrogliotoxin and 5a,6-didehydrogliotoxin on Gleospora anthracnose and Alternaria were determined by filter paper method.
[0021] Compounds 6-deoxy-5a, 6-didehydrogliotoxin and 5a, 6-didehydrogliotoxin were dissolved in DMSO and diluted to a concentration of 2 mg / mL.
[0022] Insert the phytopathogenic fungus cakes with a diameter of 4 mm in the center of the plate containing PDA medium, place 4 pieces of filter paper evenly around the cake, and drop 5 μL of extract on the filter paper as a sample group, replace the compound with DMSO The extract was used as blank control. Place in an incubator at 28°C for 3 to 5 days, record the growth diameter of the bacteria cake with the cross measurement method, and calculate the inhibition rate according to the following formula.
[0023] Inhibition rate (%)=(1-sample group pure growth amount / control group pure growth amount)×100%
[0024] The experimental results are shown ...
Embodiment 2
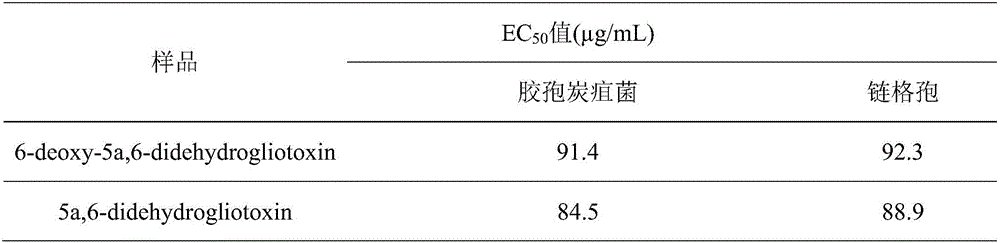
[0029] The EC of compounds 6-deoxy-5a, 6-didehydrogliotoxin and 5a, 6-didehydrogliotoxin against plant pathogenic fungi G. anthracnose and Alternaria were determined by filter paper method 50 value.
[0030] Compounds 6-deoxy-5a,6-didehydrogliotoxin and 5a,6-didehydrogliotoxin were diluted to 800, 400, 200, 100, 50, 25, 12.5 μg / mL with DMSO. Insert the phytopathogenic fungus cakes with a diameter of 4 mm in the center of the plate containing the PDA medium, place 4 pieces of filter paper evenly around the cake, and drop 5 μL of extract on the filter paper, and replace the sample extract with DMSO. Blank control. Place in a constant temperature box at 28°C for 3 to 5 days, record the growth diameter of the bacteria cake with the cross measurement method, calculate the inhibition rate, and use SigmaPlot 10.0 software to calculate the EC 50 value. The experimental results are shown in Table 2:
[0031] Table 2: EC of compounds against phytopathogenic fungi 50 value
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com