Propylgallate injection, and preparation method and application of propylgallate injection
A technology of injection and propyl gallate, which is applied in the fields of blood diseases, medical formulas, extracellular fluid diseases, etc., can solve problems such as the instability of propyl gallate injection, and achieve stability, small growth of related substances, The effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
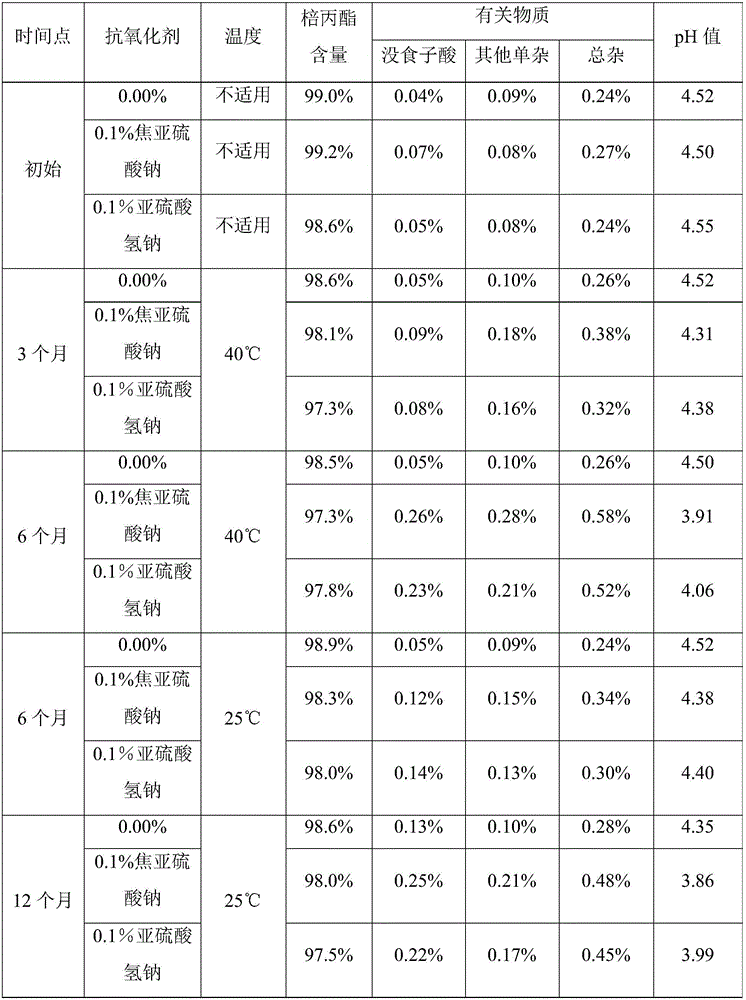
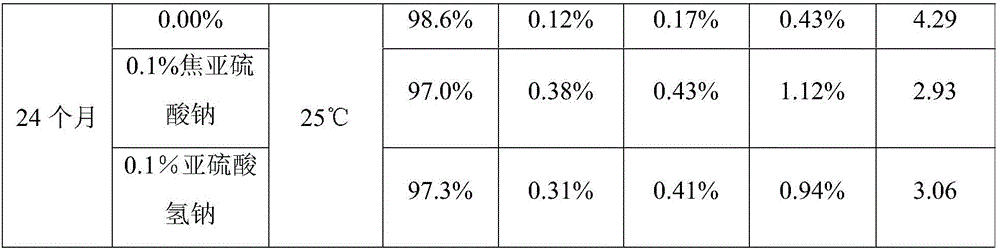
[0026] Preparation of Propylgallate Injection
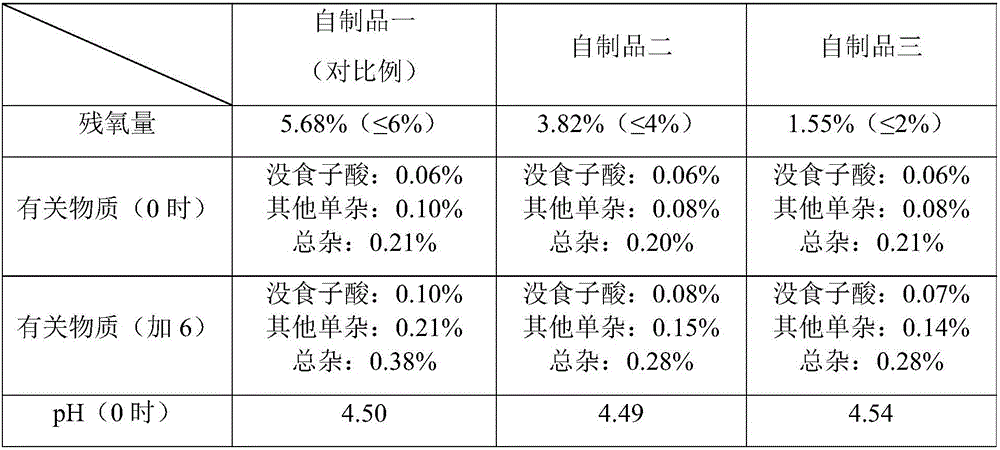
[0027] Add 30 L of propylene glycol, 0.4 g of edetate disodium to about 50 L of degassed water for injection, then add 1.2 kg of propyl gallate and mix. Phosphate buffer was added to adjust the pH to about 4.5. Sufficient degassed water for injection was added to make a 1.2% solution (total volume 100 L). Exposure to air was minimized by displacing oxygen with nitrogen. Pass the solution through a 0.2 micron sterile filter. Put the product into a vial or ampoule that minimizes the exposure of oxygen by replacing it with nitrogen to obtain an injection with a residual oxygen content of about 1.5%, and sterilize it at 121°C for 15 minutes.
Embodiment 2
[0029] Preparation of Propylgallate Injection
[0030] Add 40 L of propylene glycol, 0.4 g of edetate disodium to about 50 L of degassed water for injection, then add 1.2 kg of propyl gallate and mix. Phosphate buffer was added to adjust the pH to about 4.5. Sufficient degassed water for injection was added to make a 1.2% solution (total volume 100 L). Exposure to air was minimized by displacing oxygen with nitrogen or other pharmaceutically inert gas. Pass the solution through a 0.2 micron or other sterile filter. Put the product into a vial or ampoule that minimizes the exposure of oxygen by substituting nitrogen or other pharmaceutical inert gas to obtain an injection with a residual oxygen content of about 2%, and sterilize it at 121°C for 15 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com