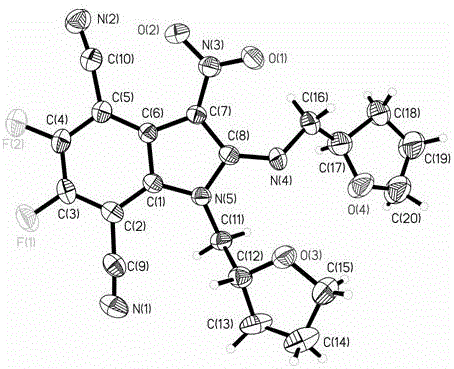
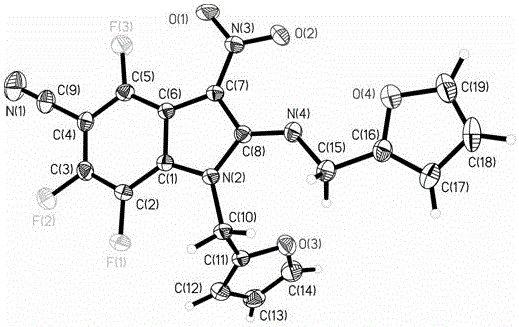
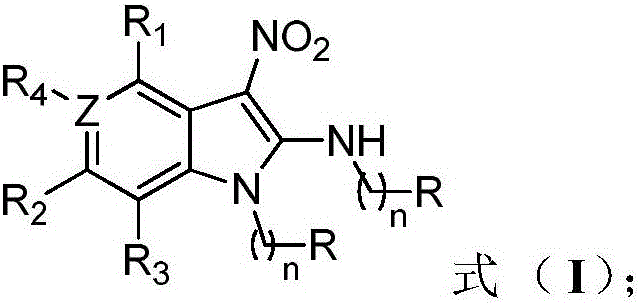
Fully-substituted 3-nitroindole compound as well as intermediate compound, preparation method and application of fully-substituted 3-nitroindole compound
A nitroindole compound and a fully substituted technology, applied in the field of medicinal chemistry, can solve the difficult and complex problems of fully substituted indole compounds, and achieve the effects of high yield, simple operation, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1: Synthesis of 1,1-bis((4-methoxybenzyl)amino)-2-nitroethylene (compound 1): 1,1-bis(methylmercapto)-2-nitroethylene ( 1.65g, 10mmol) was dissolved in 80ml of ethanol, stirred for 2 minutes, another raw material arylamine compound 4-methoxybenzylamine (3.43g, 25mmol) was added, heated to reflux for 12h, a white solid was precipitated, and the solid was Suction filtration and washing to obtain the target material (1), 1,1-bis((4-methoxybenzyl)amino)-2-nitroethylene (compound 1), yield 92%, melting point: 226.8-227.2 ℃.
[0103] Proton NMR spectrum (deuterated dimethyl sulfoxide, Bruker AM 600 instrument): δ=3.76(s, 3H, CH 3 ),3.75(s,3H,CH 3 ),4.29(s,2H,CH 2 ),4.51(s,2H,CH 2), 6.41 (s, 1H, CH), 6.91–7.30 (m, 8H, ArH), 7.89 (s, 1H, NH), 10.31 (s, 1H, NH).
Embodiment 2
[0104] Example 2: Synthesis of 1,1-bis((4-methylphenethyl)amino)-2-nitroethylene (compound 2): 1,1-bis(methylmercapto)-2-nitroethylene ( 1.65g, 10mmol) was dissolved in 50ml of ethanol, stirred for 2 minutes, another raw material arylamine compound 4-methylphenethylamine (3.38g, 25mmol) was added, heated to reflux for 24h, a white solid was precipitated, and the solid was Suction filtration and washing to obtain the target raw material (2), 1,1-bis((4-methylphenethyl)amino)-2-nitroethylene (compound 2), yield 90%, melting point: 215.3-215.7 ℃.
[0105] Proton NMR spectrum (deuterated dimethyl sulfoxide, Bruker AM 600 instrument): δ=2.25–2.29(m,6H,CH 3 ),2.74–2.77(m,4H,CH 2 ),3.36(s,4H,NHCH 2 ), 6.55 (s, 1H, CH), 7.11-7.14 (m, 8H, ArH), 7.19 (s, 1H, NH), 10.10 (s, 1H, NH).
Embodiment 3
[0106] Example 3: Synthesis of 1,1-bis((4-fluorophenethyl)amino)-2-nitroethylene (compound 3): 1,1-bis(methylmercapto)-2-nitroethylene (1.65 g, 10mmol) was dissolved in 60 ml of ethanol, stirred for 2 minutes, another raw material arylamine compound 4-fluorophenethylamine (2.78g, 20mmol) was added, heated to reflux for 18h, a white solid was precipitated, and the solid was filtered by suction , and washed to obtain the target material (3), 1,1-bis((4-fluorophenethyl)amino)-2-nitroethylene (compound 3), with a yield of 90% and a melting point of 159.2-159.6°C.
[0107] Proton NMR spectrum (deuterated dimethyl sulfoxide, Bruker AM 600 instrument): δ=2.79–2.82(m,4H,CH 2 ),3.35–3.43(m,4H,CH 2 ), 6.57 (s, 1H, CH), 7.12-7.30 (m, 8H, ArH), 7.30 (br, 1H, NH), 10.12 (br, 1H, NH).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com