Application of fingolimod hydrochloride to preparation of medicine for treating systemic lupus erythematosus encephalopathy
A technology of fingolimod hydrochloride and lupus erythematosus, which is applied in the field of medicine, can solve the problems that the treatment of lupus encephalopathy has not yet been reported, and achieve the effects of convenient administration, integrity protection, and improvement of behavioral symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
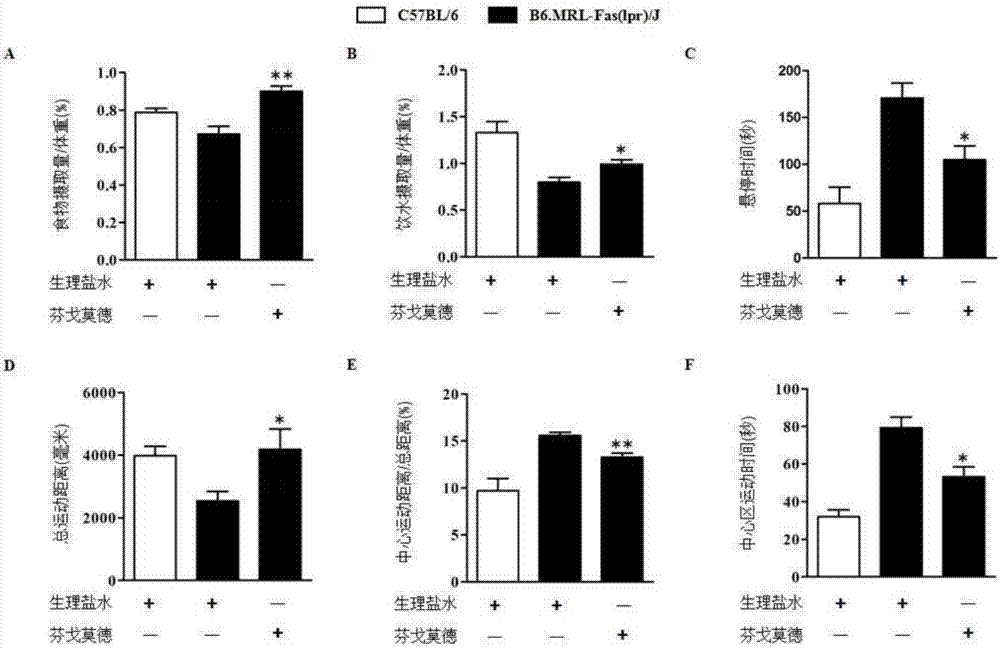
[0016] FTY720 treatment improves the behavior of B6.MRL-Fas(lpr) / J mice
[0017] 1. Method: The source of the drug needed for the experiment: FTY720 was purchased from Cayman Chemical Company. The mouse behavior experiment used open field experiment, tail suspension experiment and feeding experiment. C57BL / 6 female mice and B6.MRL-Fas(lpr) / J female mice aged about 8 weeks were divided into 3 groups: C57BL / 6 group, B6.MRL-Fas(lpr) / J group, B6 .MRL-Fas(lpr) / J+FTY720 group. The administration method of the B6.MRL-Fas(lpr) / J+FTY720 group is as follows: B6.MRL-Fas(lpr) / J mice were given 1mg / kg / day FTY720 by intragastric administration from the age of 8 weeks, three times a week , the other two groups were replaced with normal saline, and the feeding experiment, drinking water experiment, tail suspension experiment and open field experiment were carried out at about 20 weeks, and the experimental results were analyzed by SPSS statistical software.
[0018] 2. Results: see figure ...
Embodiment 2
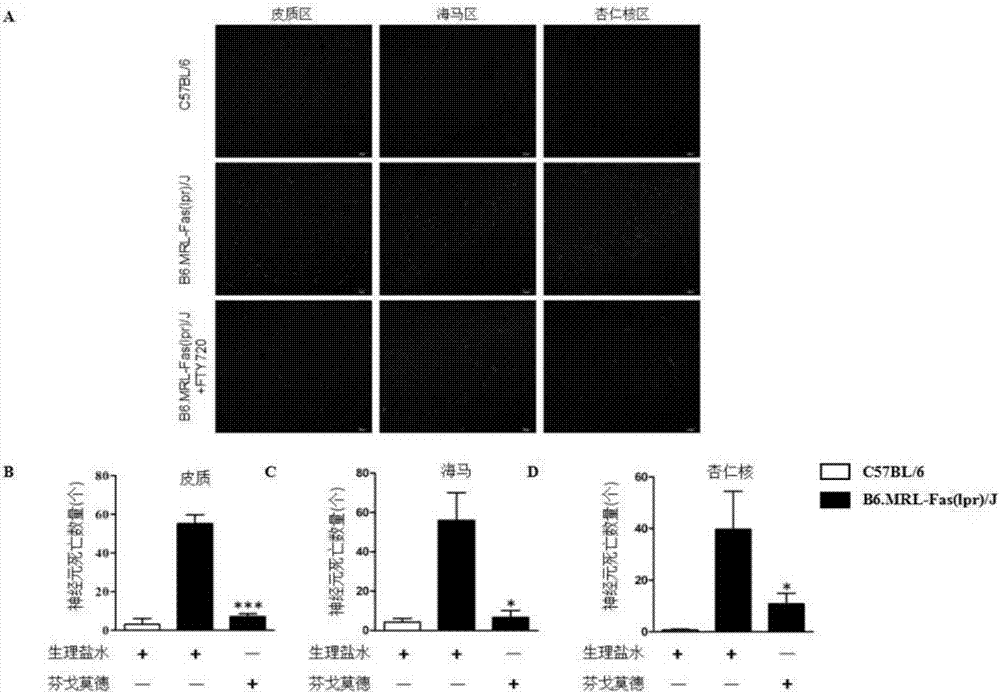
[0033] Effects of FTY720 on Neuronal Apoptosis in Brain Tissue of B6.MRL-Fas(lpr) / J Mice
[0034] 1. Method: Sources of medicines required for the experiment: Neuron apoptosis kit Fluoro-Jade B was purchased from ATT Bioquest. The mice were grouped as described in Example 1, and at 20 weeks, they were sacrificed to obtain brain tissue, stained for neuronal apoptosis, and observed under a fluorescent microscope (see procedure description).
[0035] 2. Results: see figure 2 , After FTY720 treatment, although the number of apoptotic neurons in the hippocampus of the mouse brain tissue decreased, there was no significant difference compared with the untreated group, while the apoptotic number of neurons in the amygdala area decreased significantly compared with the untreated group .
[0036] 3. Step description: Fluoro-Jade B staining
[0037] a. Mice were anesthetized at the age of 20 weeks, and their limbs were fixed on a flat plate. The chest cavity was opened to expose the...
Embodiment 3
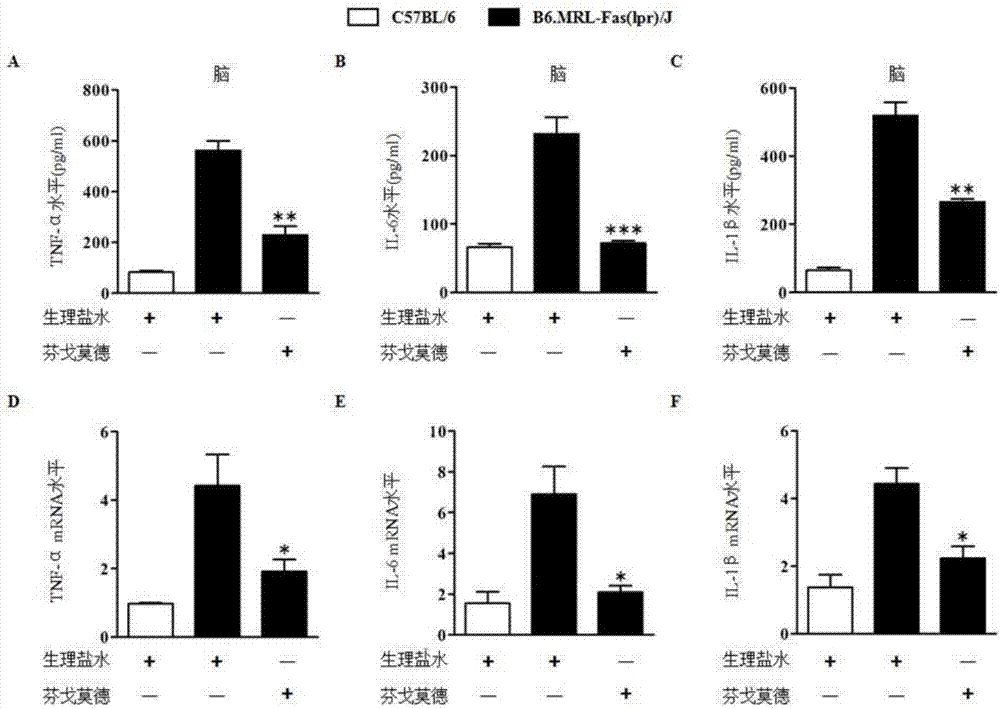
[0046] Effects of FTY720 on inflammatory factors in the brain tissue of B6.MRL-Fas(lpr) / J mice
[0047] 1. Method: The source of kits required in the experiment: TNF-α and IL-6 ELISA kits were purchased from BD Company in the United States. The mice were grouped as described in Example 1, and the brain tissues were taken for inflammatory reactions at 20 weeks. factor detection. Anesthetize the mouse and fix it on a flat plate, open the chest cavity, expose the heart, perfuse the ventricle with PBS until the tissue turns white, take the mouse brain tissue, add 500 μL PBS, centrifuge at 12,000 rpm for 5 minutes after homogenization, and take the supernatant for ELISA experiment .
[0048] 2. Results: see image 3 , B6.MRL-Fas(lpr) / J mice have an inflammatory state in the brain tissue, and the levels of TNF-α and IL-6 in the brain tissue are significantly higher than those in C57BL / 6 mice, while the levels of TNF-α and IL-6 in the brain tissue after FTY720 treatment The levels...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com