A kind of method for synthesizing cyclopentanimide
A technology of cyclopentanimide and a synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of poor appearance black and yellow quality, poor utilization effect, and high utilization cost, and achieve stable reaction process conditions, stable reaction and quality, and product quality. Stable quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
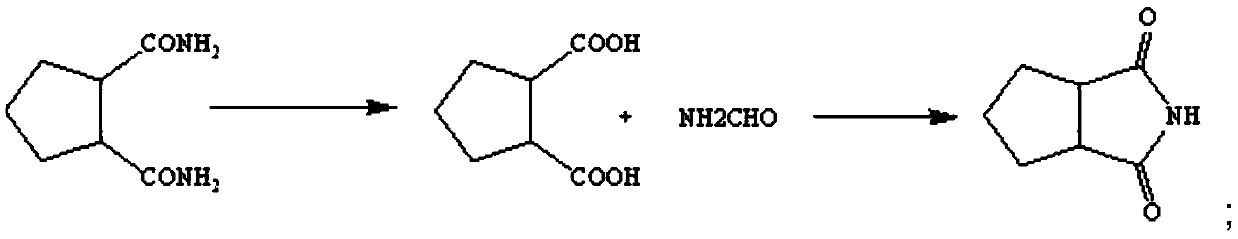
[0024] Add water and 1,2-cyclopentane diamide into the reaction kettle at a mass ratio of 1:1, dissolve, heat up to 95-105°C and reflux, start adding 40% sodium hydroxide solution dropwise, 1,2-cyclopentane The mass ratio of sodium hydroxide in glutanediamide to sodium hydroxide solution is 5:2-4, stop after dropping for about 1-3 hours, continue stirring for 30-60 minutes, when no tail gas is released, the reaction ends;
[0025] After the reaction is completed, cool down. When the temperature drops to 20-35°C, start to add concentrated hydrochloric acid for acidification. Measure the pH in the reactor. Stop when the pH is 1-2. When the acidification is complete, cool the reactor to 0-5°C and continue stirring. After 1 hour, the reactant was filtered, washed with water, and air-dried at 45-60°C to obtain 1,2-cyclopentanedioic acid;
[0026] Add 1,2-cyclopentanedioic acid and formamide into the reactor at a mass ratio of 1:2~4 and mix them. After nitrogen replacement, heat up ...
Embodiment 2
[0029] Stir mechanically in a 250mL four-necked bottle, add 60g of drinking water and 60g of 1,2-cyclopentane diamide, heat up to 100°C and reflux, start adding 90g of 40% sodium hydroxide solution dropwise, the reaction will release ammonia gas, continue stirring after the dropwise addition is complete 30min, when no tail gas is emitted, the reaction ends;
[0030] After the reaction is completed, cool down. When the temperature is 25°C, add 120g of 36% concentrated hydrochloric acid dropwise for acidification. Measure the pH. When the pH is 2, the acidification is complete. Stop the dropwise addition. After cooling down to 0°C in an ice bath, continue to stir for 1 hour and filter. Washed with water and blown dry at 55°C to obtain 57.3g of 1,2-cyclopentanedioic acid with a yield of 95.5%;
[0031] Mix 50g of 1,2-cyclopentanedioic acid and 20g of formamide, replace with nitrogen, and raise the temperature to an internal temperature of 150°C for reaction. During the reaction, ...
Embodiment 3
[0034] Stir mechanically in a 250mL four-neck bottle, add 60g of drinking water and 60g of 1,2-cyclopentane diamide, dissolve, heat up to 95°C and reflux, add 100g of 40% sodium hydroxide solution dropwise, and continue stirring for 60min after the addition is complete. When the tail gas is released, the reaction ends;
[0035] After the reaction is completed, cool down. When the temperature is 35°C, add 120g of 36% concentrated hydrochloric acid dropwise for acidification. When the pH of the mixed solution is measured to be 1-2, the acidification is completed. After cooling down to 5°C in an ice bath, continue stirring for 1 hour, filter, and wash with water for 60 ℃ blast drying to obtain 58g of 1,2-cyclopentadioic acid with a yield of 96.7%;
[0036] Mix 50g of 1,2-cyclopentanedioic acid and 28g of formamide, replace with nitrogen, and raise the temperature to an internal temperature of 200°C to react. During the reaction, the low boilers are distilled out. During the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com