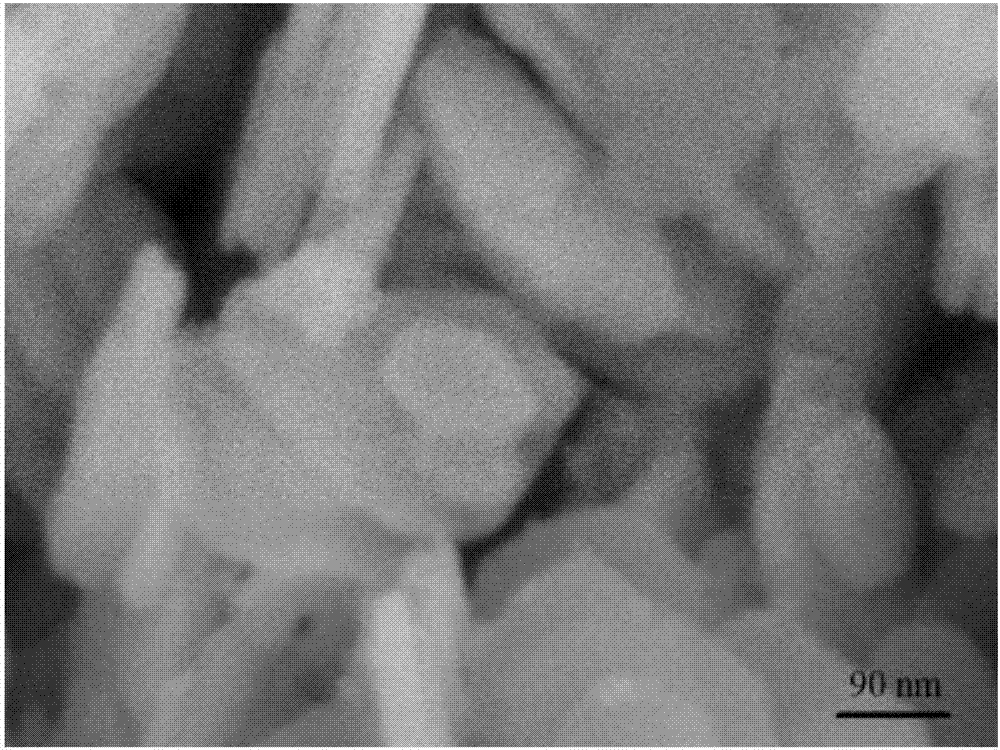
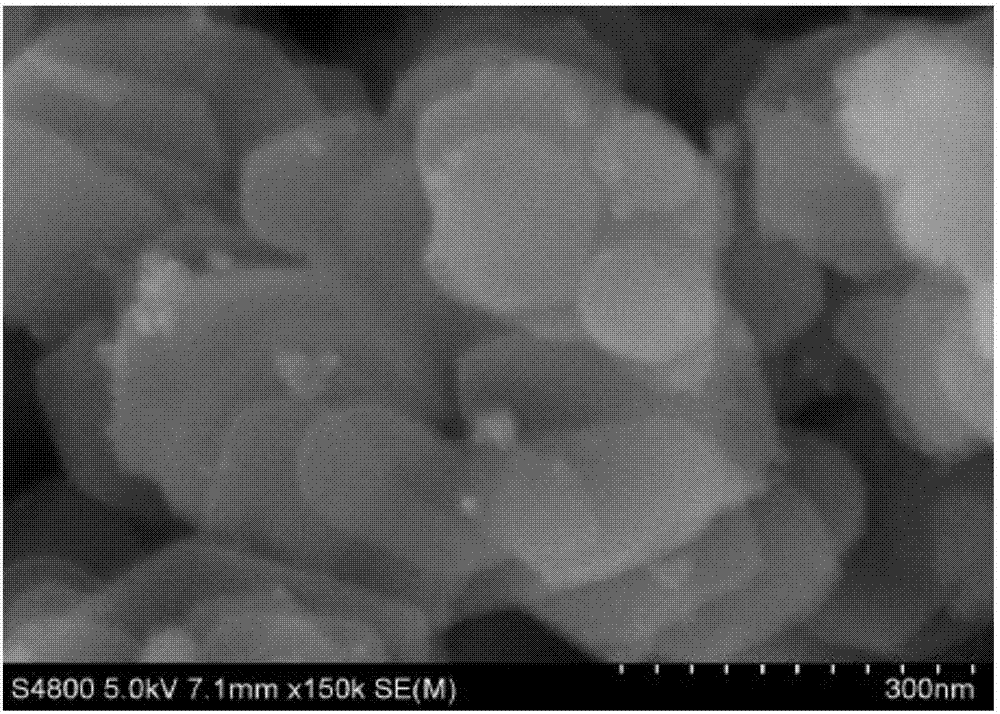
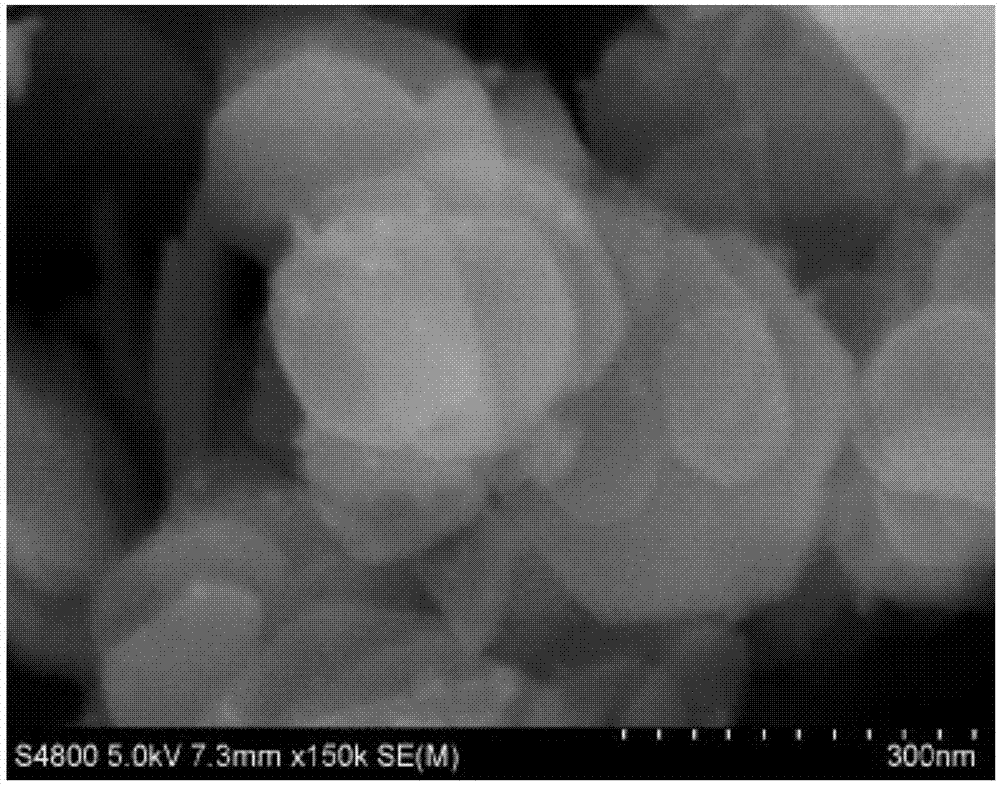
Two-dimensional ultrathin double-metal hydroxide nanosheet and preparation method thereof
A hydroxide and bimetal technology, applied in the field of two-dimensional ultra-thin bimetal hydroxide nanosheets and its preparation, can solve the problems of restricting large-scale production, complex process, high cost, etc., and achieve good oxygen evolution performance and operation Simple, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. The preparation of bulk layered cobalt-iron hydroxide comprises the following steps:
[0043] (1), 0.3mol Fe(NO 3 ) 3 9H 2 O and 0.9mol Co(NO 3 ) 2 ·6H 2 O (the molar ratio of cobalt to iron is 3:1) mixed solution is prepared with deionized water as solvent; (2), 1.92mol NaOH and 0.8mol NaOH 2 CO 3 The mixed solution was prepared with deionized water as the solvent; (3) 30 mL of each of the above two mixed solutions were added to a 100 mL beaker at the same time, and after stirring for 15 min, transferred to a 100 mL stainless steel reaction kettle, and after hydrothermal reaction at 80 ° C for 48 h, the natural Cool to room temperature. Washing with water and ethanol three times respectively, suction filtering and drying in a vacuum oven at 60°C for 10 hours to obtain the bulk layered cobalt-iron hydroxide;
[0044] 2. Treating the bulk layered cobalt-iron hydroxide with argon plasma to prepare two-dimensional ultra-thin cobalt-iron hydroxide nanosheets.
...
Embodiment 2
[0052] 1. The preparation of bulk layered cobalt-iron hydroxide comprises the following steps:
[0053] (1), 0.3mol Fe(NO 3 ) 3 9H 2 O and 0.9mol Co(NO 3 ) 2 ·6H 2 O (the molar ratio of cobalt to iron is 3:1) mixed solution is prepared with deionized water as solvent; (2), 1.92mol NaOH and 0.8mol NaOH 2 CO 3 The mixed solution was prepared with deionized water as the solvent; (3) 30 mL of each of the above two mixed solutions were added to a 100 mL beaker at the same time, and after stirring for 15 min, transferred to a 100 mL stainless steel reaction kettle, and after hydrothermal reaction at 80 ° C for 48 h, the natural Cool to room temperature. Wash with water and ethanol three times respectively, filter with suction and dry in a vacuum oven at 60° C. for 10 h to obtain the bulk layered cobalt-iron hydroxide.
[0054] 2. Treating the bulk layered cobalt-iron hydroxide with nitrogen plasma to prepare two-dimensional ultra-thin cobalt-iron hydroxide nanosheets.
[00...
Embodiment 3
[0062] 1. The preparation of bulk layered cobalt aluminum hydroxide comprises the following steps:
[0063] (1), 0.3mol Al(NO 3 ) 3 9H 2 O and 0.9mol Co(NO 3 ) 2 ·6H 2 O (the molar ratio of cobalt to aluminum is 3:1) mixed solution is prepared with deionized water as solvent; (2), 1.92mol NaOH and 0.8mol NaOH 2 CO 3 The mixed solution was prepared with deionized water as the solvent; (3), 30 mL of each of the above two mixed solutions were added to a 100 mL beaker at the same time, after stirring for 15 minutes, transferred to a 100 mL stainless steel reaction kettle, and after hydrothermal reaction at 100 °C for 24 hours, Naturally cool to room temperature. Wash with water and ethanol three times respectively, filter with suction and dry in a vacuum oven at 60° C. for 10 h to obtain the bulk layered cobalt aluminum hydroxide.
[0064] 2. Treating the bulk layered cobalt aluminum hydroxide with argon plasma to prepare two-dimensional ultrathin cobalt aluminum hydroxide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com