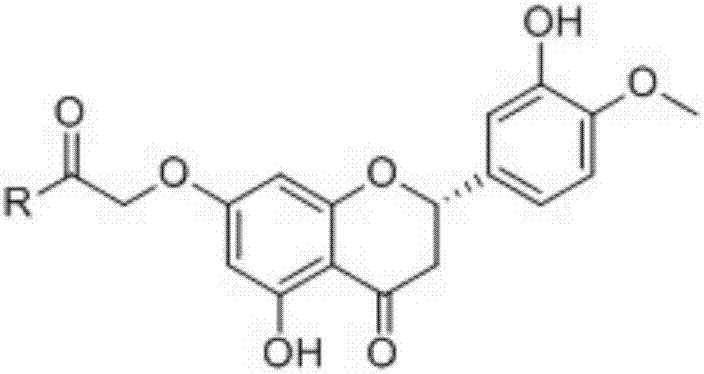
Amide group substituted hesperetin derivatives, preparation method thereof and application of derivatives as anti-AD (anti-Alzheimer's disease) drugs
A technology of hesperetin and derivatives, applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve problems such as no effective treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
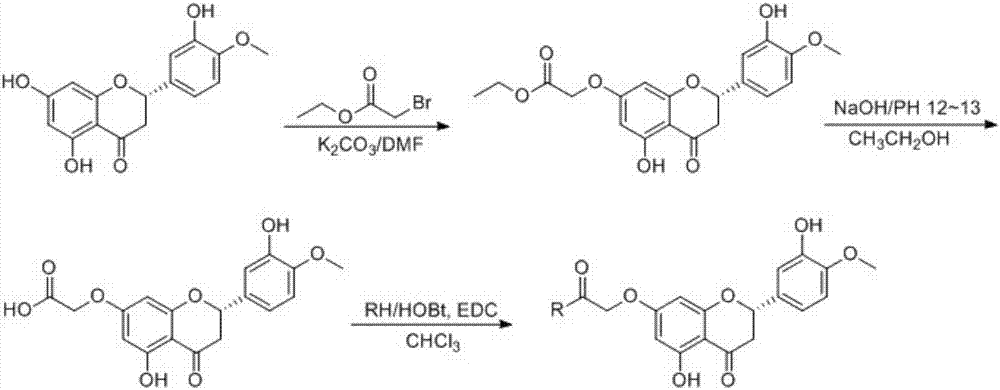
[0028] Embodiment one: the synthesis of compound 3
[0029] compound 2, hesperetin (5.72 g, about 0.02 mol) was dissolved in 100 mL of anhydrous DMF, 1.5 times the molar amount of K2CO3 was added, followed by 2 times the amount of ethyl bromoacetate, and stirred at room temperature for 20 min. Terminate the reaction, extract with ethyl acetate, repeatedly extract with saturated brine, wash away DMF, dry, recover ethyl acetate under reduced pressure, concentrate to oil, add water, precipitate a yellow solid, recrystallize with methanol and dichloromethane successively, White crystals were obtained in 68% yield,
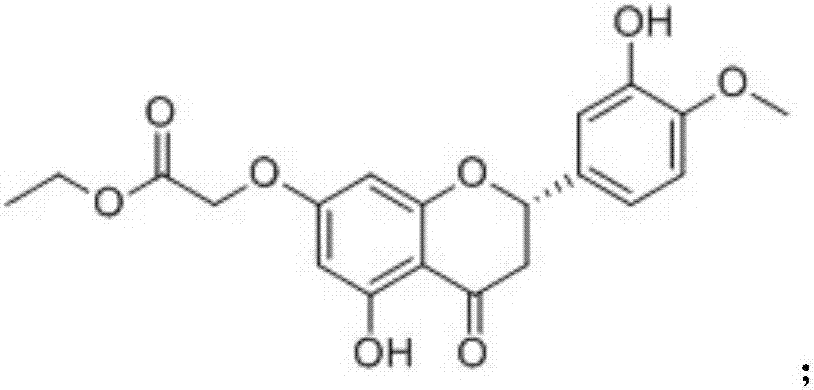
[0030] The chemical formula of compound 3 is as follows:
[0031]
Embodiment 2
[0032] Embodiment two: the synthesis of compound 5a
[0033] Compound 3 (3.72g, 10mmol), dissolved in ethanol, add 10% NaOH dropwise, adjust the pH to about 12 to 13, react for 2 hours, add dilute hydrochloric acid to adjust the pH to about 2 to 3, stop the reaction, add water to precipitate, and filter with suction The crude light yellow solid compound 4 was obtained. Take 0.33 g of the crude product of compound 4, dissolve it in chloroform, add 3 times the amount of benzylamine, add appropriate amount of HOBT and EDC hydrochloride, and stir overnight at room temperature. Stop the reaction, transfer the reaction solution to an extraction bottle, adjust the pH to a weak acid, wash with saturated brine (40mL×3), dry the organic phase with anhydrous calcium chloride, filter, and distill the filtrate under reduced pressure to obtain a light yellow oil. A white solid 5a was obtained by silica gel column chromatography [V (chloroform): V (petroleum ether) = 3: 1]. 1 H NMR (400MHz...
Embodiment 3
[0036] Embodiment three: the synthesis of compound 5b
[0037] The method was the same as in Example 2, except that p-chlorobenzylamine was used instead of benzylamine to obtain compound 5b as a white solid. 1 H NMR (400MHz, CDCl 3)δ11.98(s,5-OH),7.33–7.28(m,2H,Ar-H),7.25–7.20(m,2H,Ar-H),7.03(d,J=2.0Hz,1H,2 '-H),6.92(dd,J=8.4,1.9Hz,1H,5'-H),6.88(d,J=8.3Hz,1H,4'-H),6.74(t,J=5.7Hz, 1H,NH),6.07(d,J=2.4Hz,1H,8-H),6.04(d,J=2.4Hz,1H,6-H),5.34(dd,J=12.8,3.1Hz,1H, 2-H),4.53(s,2H,C=OCH 2 O), 4.50(d, J=6.1Hz, 2H, NCH 2 ),3.92(s,3H,OCH 3 ), 3.09(dd, J=17.2, 12.8Hz, 1H, 3-H), 2.82(dd, J=17.2, 3.1Hz, 1H, 3-H).TOF-HRMS m / z: 509.0977[M+Na ] + ,
[0038] The chemical formula of compound 5b is as follows:
[0039]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com