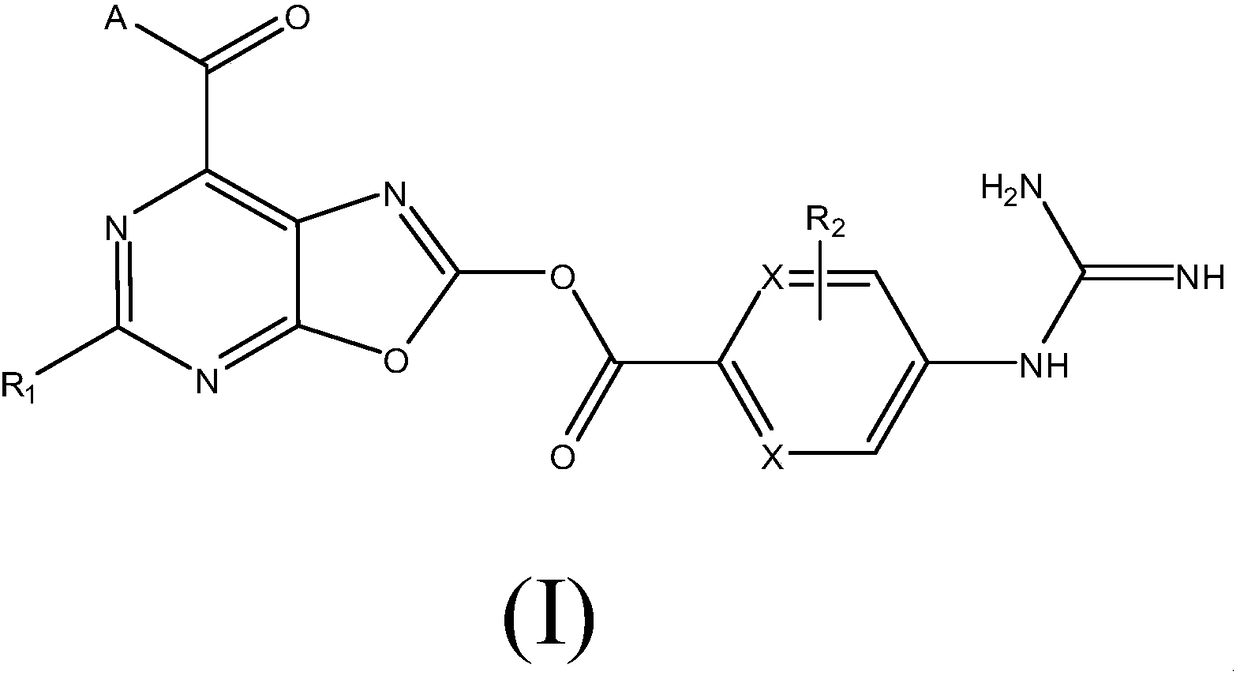
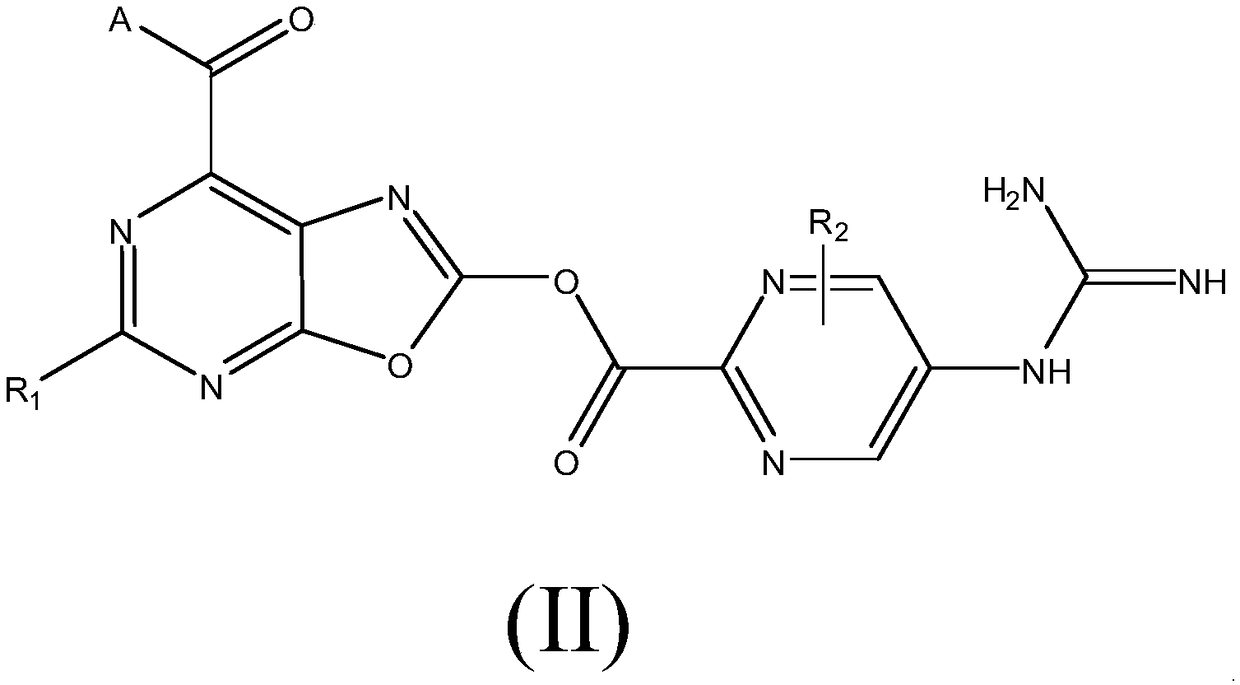
A kind of medicine for preventing and treating acute kidney injury and its preparation method and application
A pharmaceutical and compound technology, applied in the field of oxazolopyrimidine compounds, can solve the problems of cell surface S1P1 reduction, lymphocyte reduction, etc., and achieve therapeutically applicable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
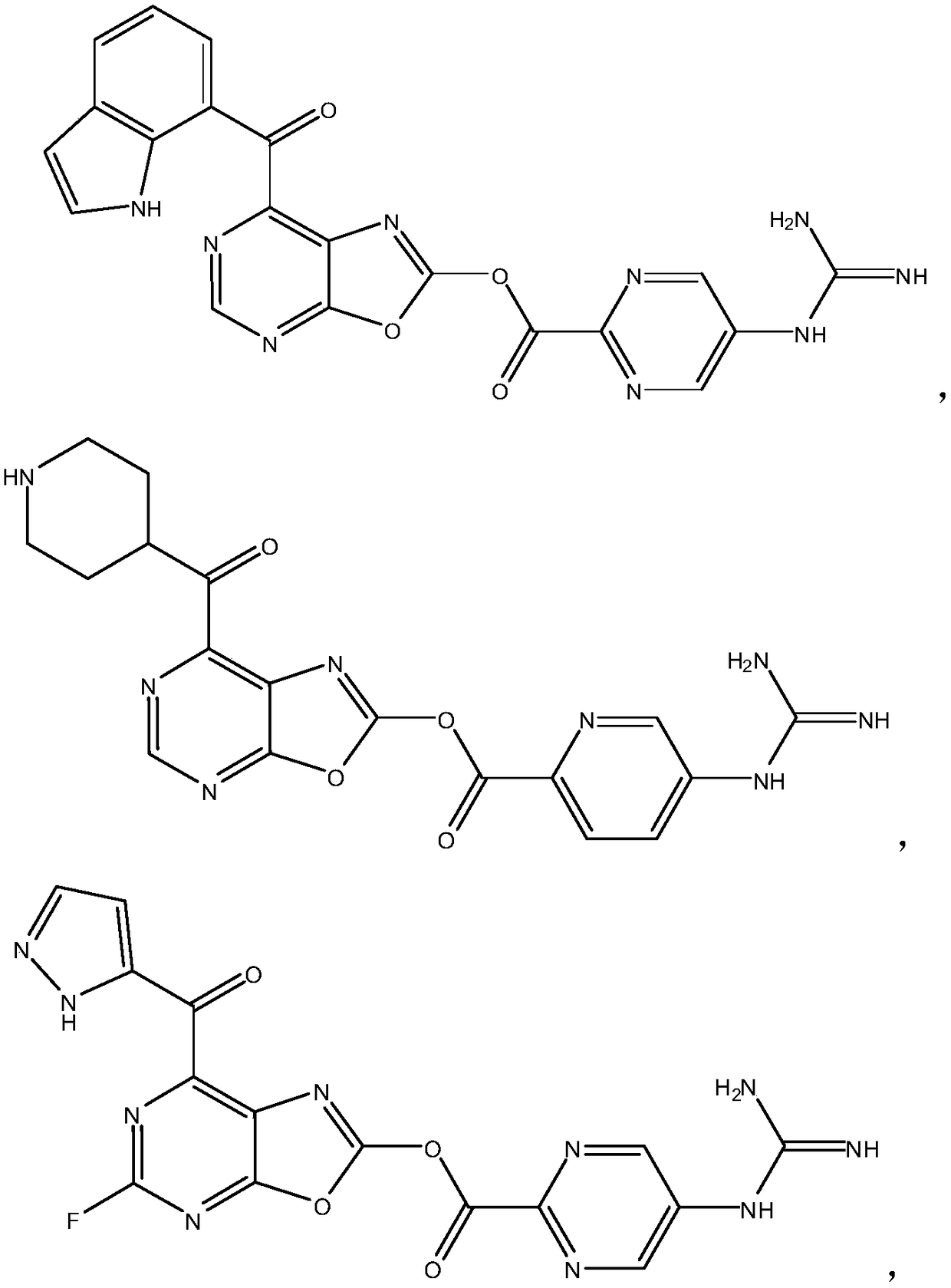
[0042] Example 1: 7-(1H-indole-7-carbonyl)oxazol[5,4-d]pyrimidin-2-yl 5-guanidinopyrimidine-2-carboxylate (Compound 1)
[0043]
[0044] In a three-necked flask equipped with a nitrogen protection device, a thermometer, and a reflux condenser, add 2.7g (0.015mol) of 5-guanidinopyrimidine-2-carboxylic acid and toluene (100ml) under nitrogen protection, and stir to make it dissolve. Then add 2.6g (0.016mol) of carbonyldiimidazole, heat to 80°C, and react for 2 hours, then add dropwise 2.2g (0.016mol) of oxazol[5,4-d]pyrimidin-2-ol in toluene (50ml) solution, and then heated to reflux, and reacted for 4 hours. After cooling the reaction solution, the solvent was evaporated under reduced pressure. The residue was washed with water and recrystallized with ethanol to obtain a white solid oxazol[5,4-d]pyrimidine-2- 3.9 g of 5-guanidinopyrimidine-2-carboxylate, yield 87%, ESI-MS: 301.07 [M+H] + .
[0045] Add aluminum trichloride (5.6g, 44.0mmol), toluene (100ml) into a three-nec...
Embodiment 2
[0049] Example 2: 7-(piperidine-4-carbonyl)oxazol[5,4-d]pyrimidin-2-yl 5-guanidinopyridine-2-carboxylate (Compound 2)
[0050]
[0051] According to the method of Example 1, replace 5-guanidinopyrimidine-2-carboxylic acid with 5-guanidinopyridine-2-carboxylic acid, and replace 1H-indole-7-acyl chloride with piperidine-4-acyl chloride to obtain a white solid , total yield 51%, ESI-MS: 411.15[M+H] + .
Embodiment 3
[0052] Example 3: 5-fluoro-7-(1H-pyrazole-5-carbonyl)oxazol[5,4-d]pyrimidin-2-yl 5-guanidinopyrimidine-2-carboxylate (Compound 3)
[0053]
[0054] According to the method of Example 1, replace oxazol[5,4-d]pyrimidin-2-ol with 5-fluoro-oxazol[5,4-d]pyrimidin-2-ol, and use 1H-pyrazole-5- Acid chloride replaced 1H-indole-7-acyl chloride to give a bright white solid with a total yield of 45%, ESI-MS: 413.08[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com