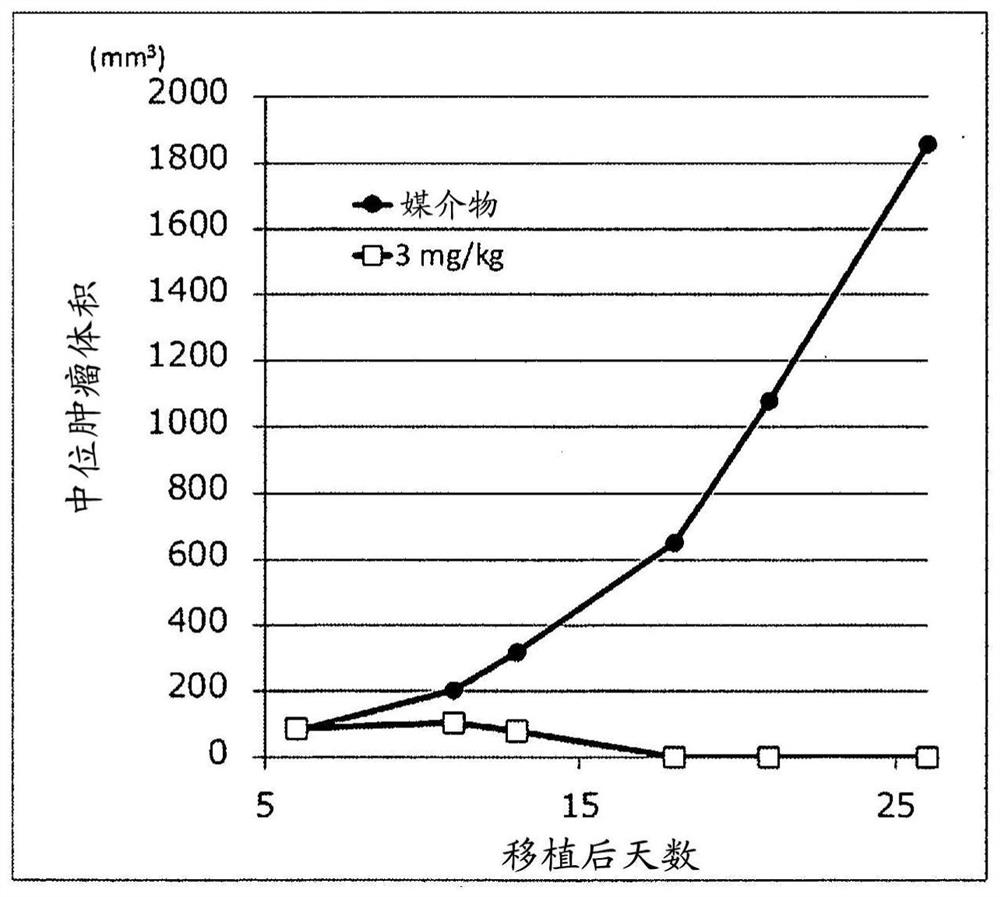
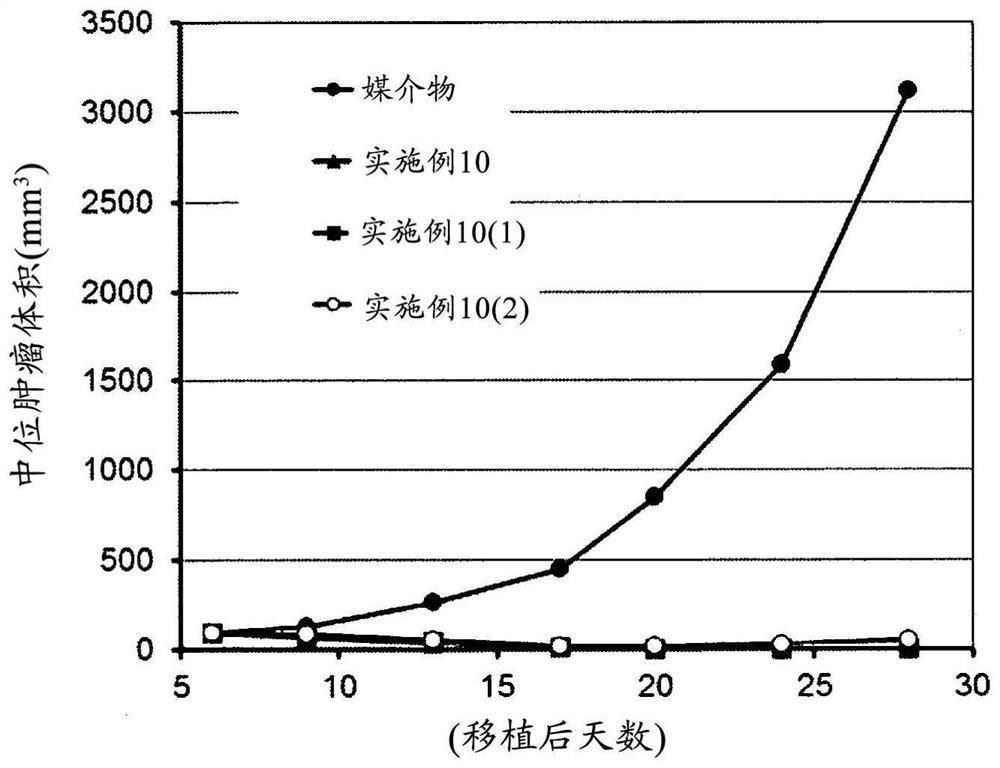
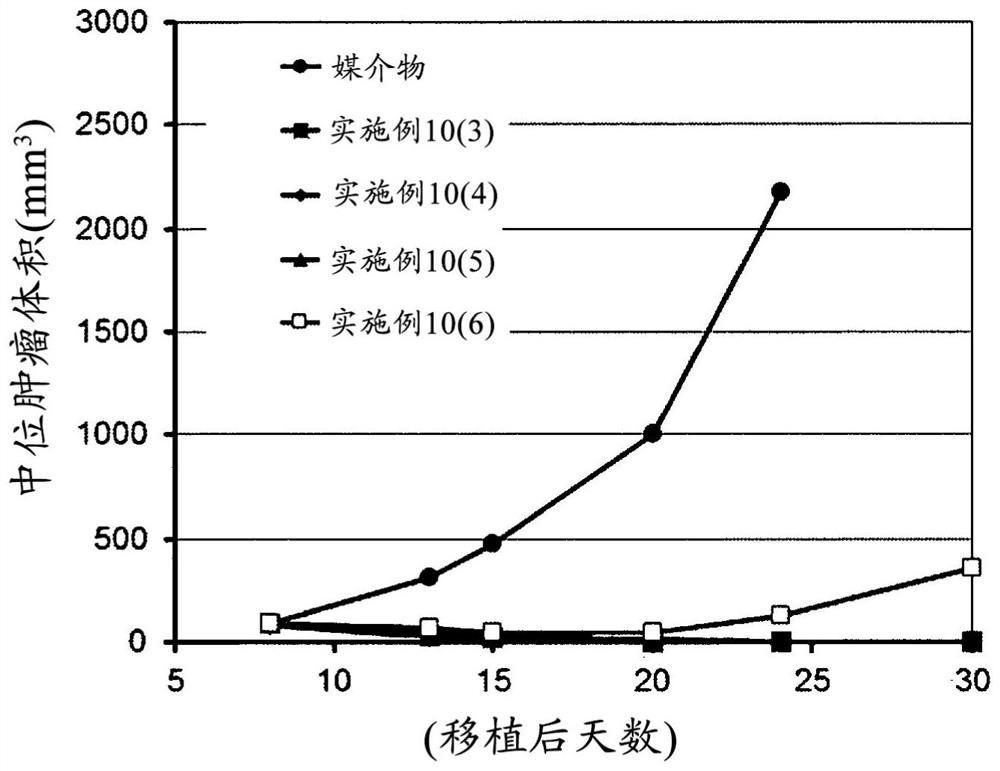
Sting-agonist compound
A compound and solvate technology, applied in the field of STING agonist compounds, can solve the problems of unreported STING agonist compounds, and achieve the effect of inhibiting progress and inhibiting recurrence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0486] Example 1: 2-amino-5-(3-amino-7-(1H-pyrazol-4-yl)isoxazolo[4,5-c]pyridin-4-yl)-4- Methyl fluorobenzoate
[0487]
[0488] Trifluoroacetic acid (4.0 mL) was added to a dichloromethane solution (4.0 mL) in which the compound produced in Reference Example 13 (388 mg) was dissolved, and the mixture was stirred at 40°C for 5 hr. To the reaction solution was added saturated sodium bicarbonate, and the mixture was extracted with ethyl acetate. The organic layer was washed with saturated brine, dried over sodium sulfate, and concentrated. The residue thus obtained was purified by silica gel column chromatography (Hi-flash SI) (hexane:ethyl acetate=90:10 to 0:100) to obtain the present compound (19.6 mg) having the following physical property values.
[0489] LCMS retention time (min): 0.59;
[0490] MS (ESI, Pos.): 369 (M+H) + ;
[0491] 1 H-NMR (CD 3 OD): δ8.86(s,1H),8.34(s,2H),8.05(d,J=8.5Hz,1H),6.66(d,J=12.5Hz,1H),3,85(s,3H ).
Embodiment 2
[0492] Example 2: 4-(4-amino-2-fluoro-5-methoxyphenyl)-7-(1H-pyrazol-4-yl)isoxazolo[4,5-c] Pyridin-3-amine hydrochloride
[0493] Under nitrogen atmosphere, 5-fluoro-2-methoxy-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)aniline (CAS No.1326283-60-6) (224 mg), bis[tri-tert-butylphosphine] palladium (65.9 mg) and 2 mol / L tripotassium phosphate aqueous solution (1.1 mL) were added to the compound dissolved in Reference Example 6 (235 mg) in 1,4-dioxane (7.1 mL), and the mixture was stirred at 110° C. for 3 hours. The reaction solution was directly purified by silica gel column chromatography (Hi-flash SI) (hexane:ethyl acetate=100:0 to 0:100) to give 4-(4-amino-2-fluoro-5-methoxyphenyl )-7-(1-(tetrahydro-2H-pyran-2-yl)-1H-pyrazol-4-yl)isoxazolo[4,5-c]pyridin-3-amine (108 mg).
[0494] Hydrochloric acid (10% methanol solution, 2.0 mL) was added to a THF solution (2.2 mL) in which the compound (108 mg) was dissolved, and the mixture was stirred at room temperature for 2 hr...
Embodiment 3
[0498] Example 3: 1-(2-amino-5-(3-amino-7-(1H-pyrazol-4-yl)isoxazolo[4,5-c]pyridine-4- Base)-4-fluorophenyl)ethan-1-one
[0499]Under a nitrogen atmosphere, borate (10.7 g), butyl bis-1-adamantylphosphine (984 mg), palladium acetate (308 mg), potassium iodide (456 mg) and 2 mol / L tripotassium phosphate aqueous solution (28 mL) was added to 1-methyl-2-pyrrolidone solution (hereinafter, abbreviated as NMP) (100 mL) in which the compound (10.0 g) produced in Reference Example 6 was dissolved, and it was The mixture was stirred at 50 to 60°C for 45 hours. After the reaction solution was cooled, insoluble matter was removed by filtration while washing with NMP. To the obtained filtrate was added tap water (240 mL) little by little, the mixture was stirred for 40 minutes, and the solid precipitated therein was collected by filtration. The solid obtained was slurry washed sequentially with acetonitrile (80 mL, twice) and methyl tert-butyl ether (80 mL, twice), then filtered an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com