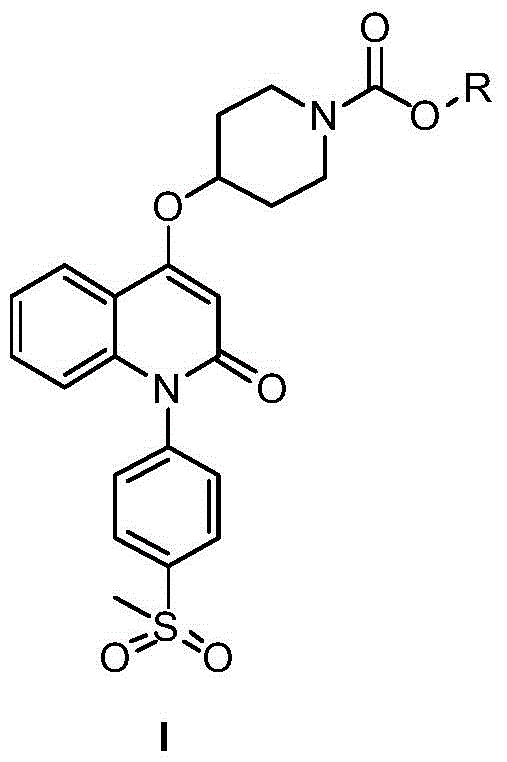
Quinolinone derivatives and their preparation methods and applications
A technology of quinolinone and its derivatives, which is applied in the field of quinolinone derivatives and their preparation, and can solve the problems of less research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
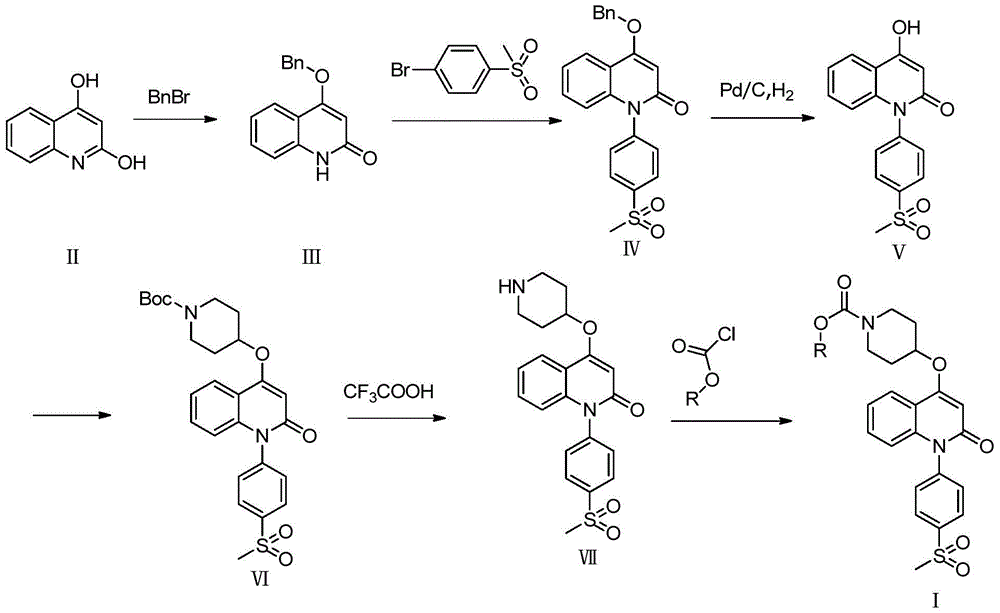
[0018] The preparation process of quinolinone derivatives of the present invention is shown in following synthetic route:
[0019]
[0020] Among them, R means C 1 -C 4 Hydrocarbyl.
Embodiment 1
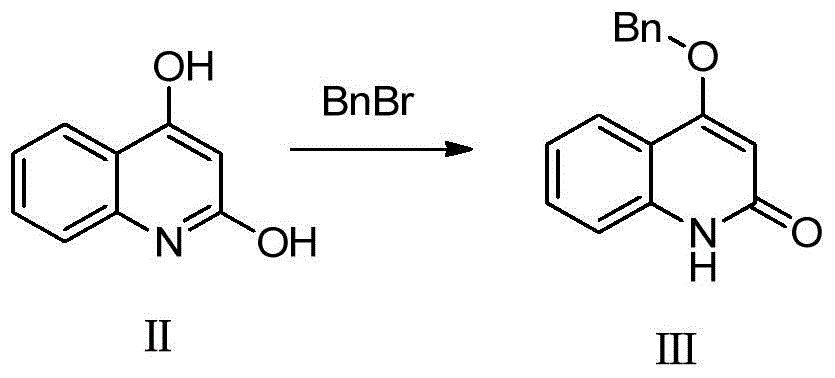
[0022] Synthetic formula III compound
[0023]
[0024] Add 10g (62.2mmol) of compound of formula II to 200mL of N,N-dimethylformamide, then add 12.88g (93.4mmol) of potassium carbonate and 7.6mL (62.2mmol) of benzyl bromide, and heat to 100°C for reaction 5 Hour. The system was poured into water, filtered, dried, and the crude product was refluxed in ethyl acetate to obtain 7.0 g of white solid, namely the compound of formula III, with a yield of 44.8%.
Embodiment 2
[0026] Synthetic formula IV compound
[0027]
[0028] 3g (11.9mmol) formula III compound, 9g (38.5mmol) p-thymphenyl bromobenzene, cuprous iodide (5.1mmol), 720mg (5.0mmol) 8-hydroxyquinoline and 4.08g (29.6mmol) potassium carbonate Dissolve in 100 mL of anhydrous dimethyl sulfoxide, under nitrogen protection, heat up to 130°C, and react for 8 hours. After the reaction was completed, it was poured into water, filtered, and the filter cake was refluxed with ethyl acetate to obtain 1.9 g of a white solid, namely compound IV, with a yield of 40%.
[0029] 1 H NMR (400MHz, CDCl 3 )δ8.18(d, J=7.9Hz, 2H), 8.09(d, J=7.9Hz, 1H), 7.59–7.31(m, 8H), 7.23(d, J=7.6Hz, 1H), 6.56( d, J=8.5Hz, 1H), 6.18(s, 1H), 5.25(s, 2H), 3.15(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com