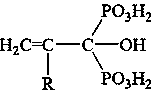
A kind of terminal ethylenically unsaturated phosphonic acid monomer and its preparation method
An unsaturated, alkenyl-terminated technology, which is applied in the field of alkenyl-terminated groups, can solve problems such as inability to undergo copolymerization reactions and limited application ranges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] In the reaction flask equipped with stirrer, reflux condenser, thermometer, dropping funnel, and heat exchange, according to the calculation of phosphorus trichloride, acrylic acid and water molar ratio is 1:3.15:1, first add 200g of acrylic acid, and then slowly Slowly add 121.1g of phosphorus trichloride and keep stirring. The time of dropping is about 1h. The temperature of the cold water bath is controlled below 40°C. ℃, after reflux reaction for 30 minutes, continue to slowly raise the temperature to 110~120 ℃, and keep at this temperature for 2 hours, until no hydrogen chloride is produced, and the hydrogen chloride gas generated during the reaction is absorbed with water. Release the supernatant, the by-product unsaturated acyl chloride can be used as an acylating reagent, the lower layer product is moved to a distillation bottle, add 15.78g of water and distill under reduced pressure for 1~2h, remove the by-product acrylic acid and residual HCl, and the reaction ...
Embodiment 2
[0028] According to phosphorus trichloride, acrylic acid and water molar ratio is 1:3.2:1 calculation, at first add 200g acrylic acid, then slowly add dropwise 119.2g phosphorus trichloride and keep stirring, other conditions are the same as embodiment 1, lower floor product shifts Add 15.63g of water to the distillation bottle for distillation under reduced pressure. After the reaction was completed, the hydroxypropenylidene diphosphonic acid of the product was obtained, and the content of the product was analyzed by the thorium nitrate rapid titration method. The calculated yield was 85.68%, and 230.19 g of water was added to form a 40% aqueous solution.
Embodiment 3
[0030] According to the calculation that the molar ratio of phosphorus trichloride, methacrylic acid and water is 1:3.15:1, first add 200g of methacrylic acid, then slowly add 101.3g of phosphorus trichloride dropwise and keep stirring, other conditions are the same as in Example 1 , the lower floor product was moved to a distillation bottle and added 13.28g of water for distillation under reduced pressure. After the reaction was over, the hydroxymethylacrylic acid diphosphonic acid was obtained. The content of the product was analyzed by the thorium nitrate rapid titration method. The calculated yield was 83.88%, and 194.15% of water was added. g into a 40% aqueous solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com