Recombinant adeno-associated virus preparation method and recombinant baculovirus
A technology of recombinant baculovirus and baculovirus, applied in the field of bioengineering, can solve problems such as poor flexibility, low versatility, and complex construction, and achieve the effect of reducing difficulty and enhancing flexibility and versatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
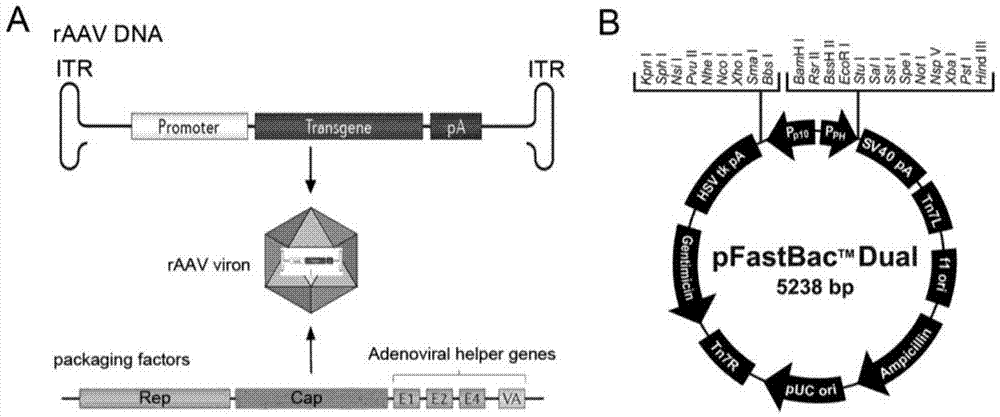
[0031] The preparation method of the recombinant adeno-associated virus provided by the invention comprises the following steps:
[0032](1) The recombinant baculovirus constructed with recombinant adeno-associated virus ITR core expression element and Cap gene or Rep gene is infected with the corresponding host packaging cell line.
[0033] The recombinant baculovirus is used to provide the ITR core expression elements and Cap gene or Rep gene required for the preparation of rAAV, and activate the baculovirus-specific promoter PH or P10 by infecting the host packaging cell line to induce the host packaging cell line to express The Rep gene or Cap gene thus assists the replication and assembly of rAAV.
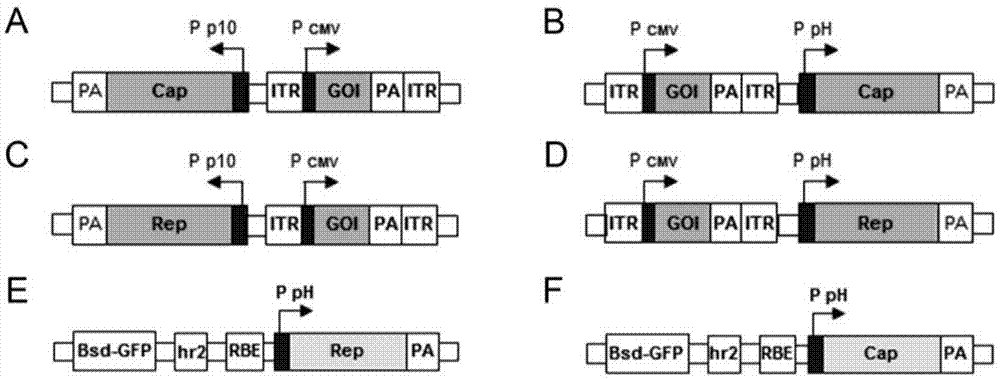
[0034] The recombinant baculovirus preferably utilizes the pFast.Bac.Dual (pFBD) shuttle vector of the Bac to Bac system (such as figure 1 B) Construction, the specific method is as follows:
[0035] a. The ITR core expression element was cloned into the P10 promoter and PH ...
Embodiment 1
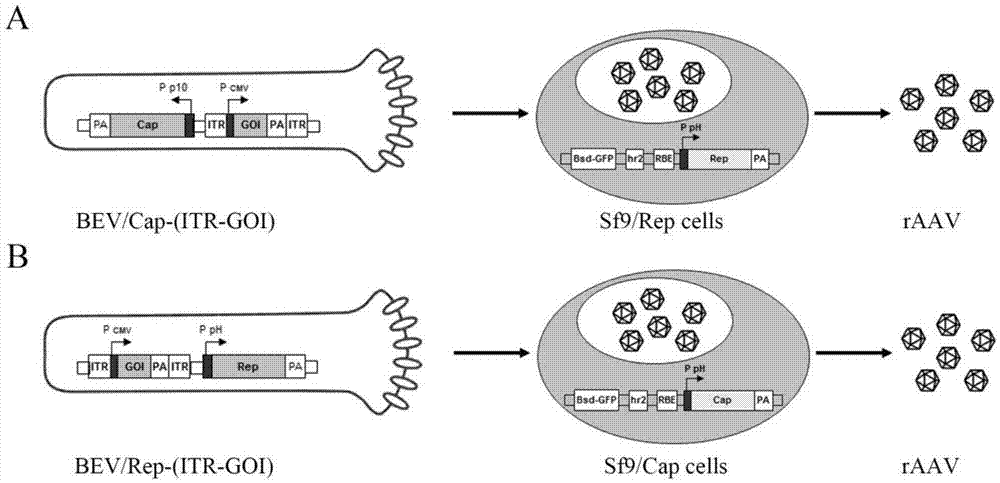
[0057] Embodiment 1: Recombinant baculovirus BEV / Cap-(ITR-GFP) infects Sf9 / Rep packaging cell line to prepare rAAV
[0058] (1) Infect the corresponding host packaging cell line with the recombinant baculovirus constructed with the recombinant adeno-associated virus ITR core expression element and the Cap gene of the corresponding serotype
[0059] The recombinant baculovirus constructed with the recombinant adeno-associated virus ITR core expression element and the Cap gene of the corresponding serotype, that is, the recombinant baculovirus BEV / Cap-(ITR-GFP), was prepared and amplified as follows:
[0060] In order to place the ITR core expression element and the Cap gene required for the preparation of rAAV into a recombinant baculovirus. We utilized the pFast.Bac.Dual (pFBD) shuttle vector in the baculovirus expression system (Bac to Bac) (eg figure 1 B). In the example, the Cap gene based on type 2 AAV was codon-optimized according to the principle of ribosome leakage sc...
Embodiment 2
[0078] Embodiment 2: Recombinant baculovirus BEV / Cap-(ITR-GFP) infects Sf9 / Rep packaging cell line to prepare rAAV
[0079] This embodiment is similar to embodiment 1, the only difference is that the recombinant baculovirus, its main component combination scheme is as follows:
[0080] LinkA-(ITR-GFP)-linkB-CapB
[0081] After infecting the Sf9 / Rep packaging cell line with the recombinant baculovirus BEV / Cap-(ITR-GFP) in this example, the prepared rAAV was purified. The yield of rAAV during the purification process is shown in Table 2. The experimental results show that the rAAV yield of a single Sf9 / Rep packaging cell can reach 7.20×104VG, and after purification by this method, the recovery rate reaches about 31.3%.
[0082] Table 2 Yield analysis of rAAV purification process
[0083]
[0084] HEK293 cells were inoculated into 96-well plates at 1×104cells / well, and infected with purified rAAV at corresponding concentration gradients 6 hours later. After 48 hours of inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com