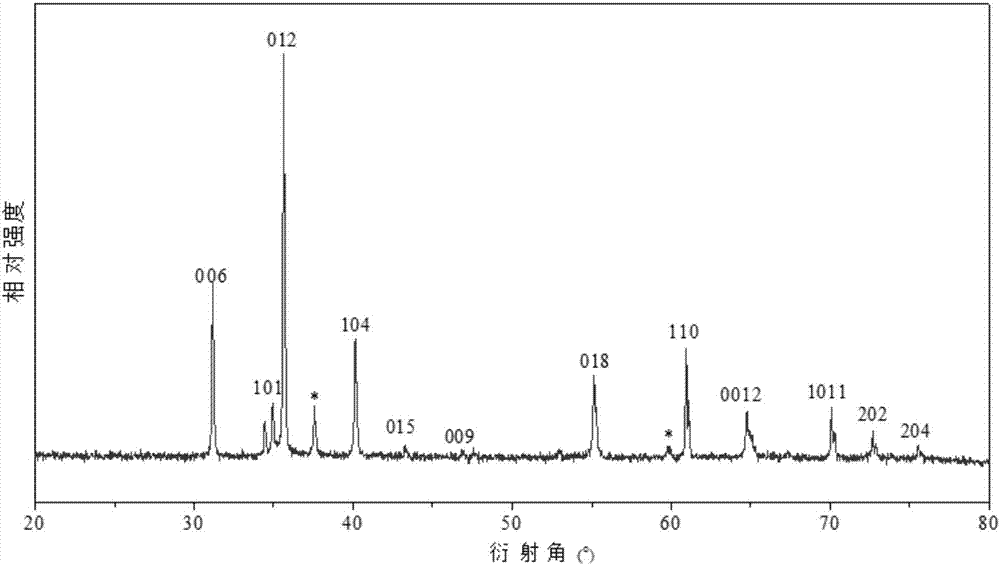
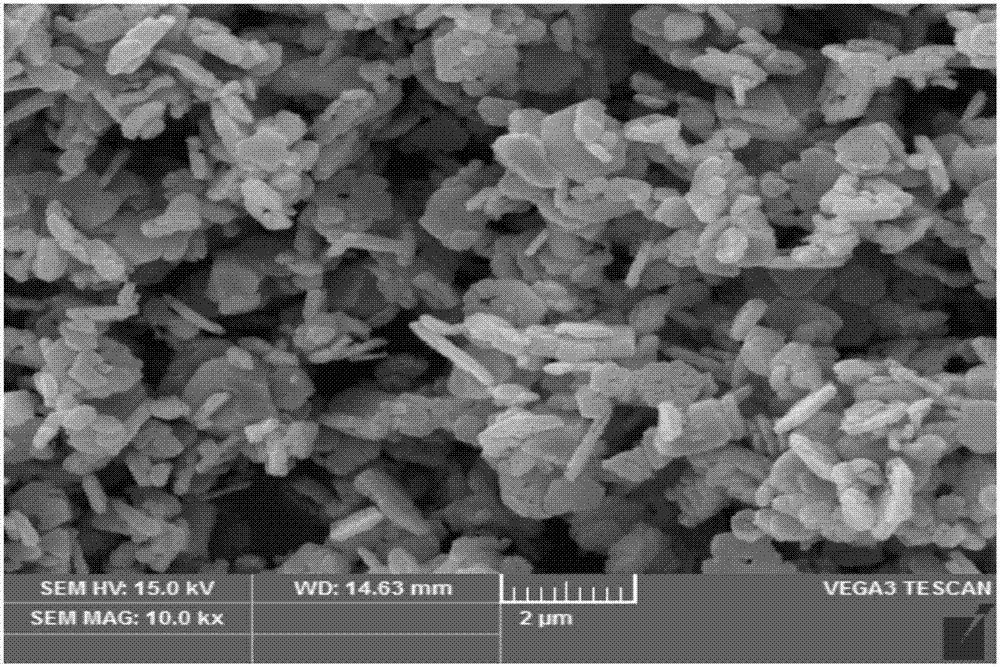
Method for preparing porous structure delafossite crystalline material
A technology of crystalline materials and porous structures, applied in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve the problems of small specific surface area and disadvantage, and achieve the effects of good repeatability, easy operation and wide source.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Dissolve 4 g of NaOH in 30 ml of deionized water and stir to form a uniform mixture.
[0036] 2) Add 0.005 mol of Cu(NO 3 ) 2 and FeSO 4 After dissolving in 10 ml of water, after the dissolution is complete, mix the two solutions, and then add to step 1) the mixture dropwise to form a slightly yellow mixture.
[0037] 3) After stirring and ultrasonicating the mixture obtained in step 2) respectively for 30 minutes, add 0.5ml of propionaldehyde, transfer it to a 100ml hydrothermal kettle, and react for 24 hours at a temperature of 160°C, and then use Wash with absolute ethanol 3 times and then wash with deionized water until neutral, dry by centrifugal, and grind.
Embodiment 2
[0039] 1) Weigh 0.25 g of PEG (polyethylene glycol with a molecular weight of 20,000) and dissolve it in 30 ml of deionized water. After the dissolution is complete, add 4 g of NaOH to dissolve in the PEG mixture and stir to form a uniform mixture.
[0040] 2) Add 0.005 mol of Cu(NO 3 ) 2 and FeSO 4 After dissolving in 10 ml of water, after the dissolution is complete, after mixing the two solutions, add dropwise to the mixture in step 1 to form a slightly yellow mixture.
[0041] 3) After stirring and ultrasonicating the mixture obtained in step 2) respectively for 30 minutes, add 0.5ml of propionaldehyde, transfer it to a 100ml hydrothermal kettle, and react for 24 hours at a temperature of 160°C, and then use Wash with absolute ethanol three times and then wash with deionized water until neutral, centrifugally dry, grind, and set aside.
[0042] 4) Place the sample obtained in step 3) in a muffle furnace, 2 Under flow protection, after calcination at 450 °C for 4 h, the...
Embodiment 3
[0045] (1) Weigh 0.4 g of PEG (molecular weight: 20,000) and dissolve it in 30 ml of deionized water. After the dissolution is complete, add 4 g of NaOH to dissolve in the PEG mixture and stir to form a uniform mixture.
[0046] (2) Add 0.005 mol of Cu(NO 3 ) 2 and FeSO 4 After dissolving in 10 ml of water, after the dissolution is complete, after mixing the two solutions, add dropwise to the mixture in step 1 to form a slightly yellow mixture.
[0047] (3) Stir and sonicate the mixture obtained in step 2 for 30 min respectively, add 0.8 ml of propionaldehyde, transfer to a 100 ml hydrothermal kettle, and react for 24 hours at a temperature of 180°C. Wash with absolute ethanol for 3 times and then with deionized water until neutral, centrifugally dry, grind, and set aside.
[0048] (4) Place the sample obtained in step 3) in a muffle furnace, 2 Under flow protection, after calcination at 500 °C for 4 h, the obtained sample is porous CuFeO 2 crystal material.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com