Ionogel forming an autosupported electrolyte film, electrochemical device incorporating same and fabrication process for ionogel
A technology of solid electrolyte and ion gel, applied in the direction of electrolyte immobilization/gelation, electrochemical generator, non-aqueous electrolyte storage battery, etc., which can solve the problems of unsuitable application, insufficient mechanical strength, separation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
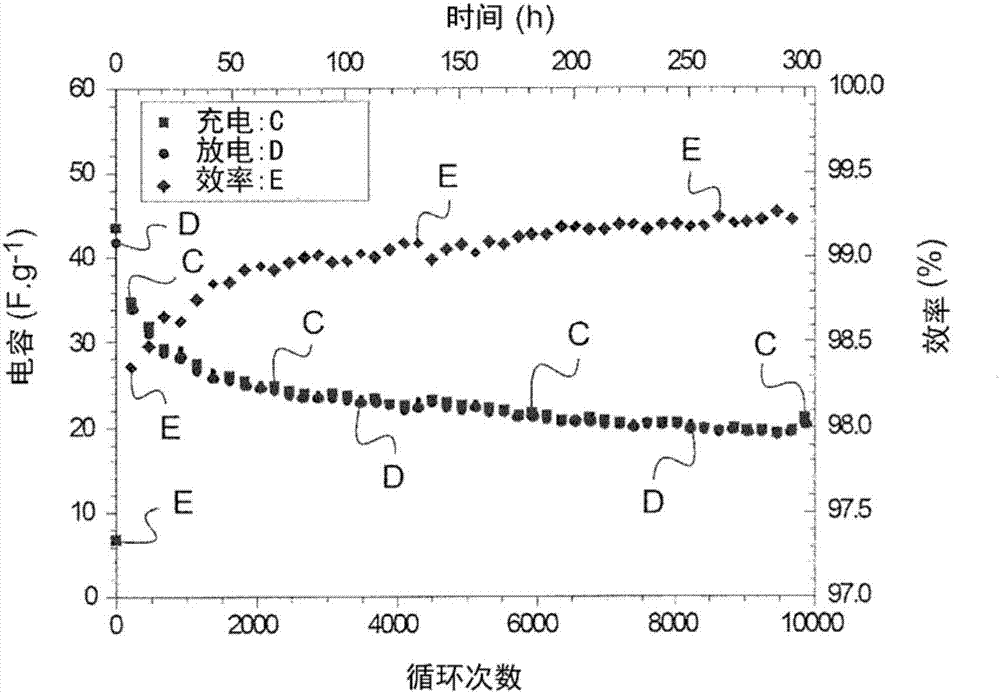
Examples
Embodiment 1
[0087] Example 1 : Production of ionomers according to the invention compared to two "control" membranes incorporating PLA not according to the invention sub-gel film
[0088] 380 mg of PLA was mixed with 2.2 mL of solvent to obtain a PLA concentration of approximately 175 g / L. The solution was stirred until the polymer was completely dissolved, ie about 2 hours.
[0089] Then 340 mg of ionic liquid (EMimTFSI) and 473 μL of silica precursor (TEOS) were added to form an ion gel [PLA / SiO 2 ] / EMimTFSI.
[0090] The solution was homogenized by magnetic stirring for 10 min. Excess formic acid (643 μL of FA (abbreviated form)) was added such that the molar ratio r=(moles of FA) / (moles of TEOS)≧8. The solution was stirred for 1 to 2 minutes.
[0091] It was then coated onto a PEN support previously cleaned with acetone. Set the coating speed to 5cm.s -1 , with a deposition height of 300 μm. The film was gelled and air dried for 24 hours, then heated at 110° C. for 1 hour. ...
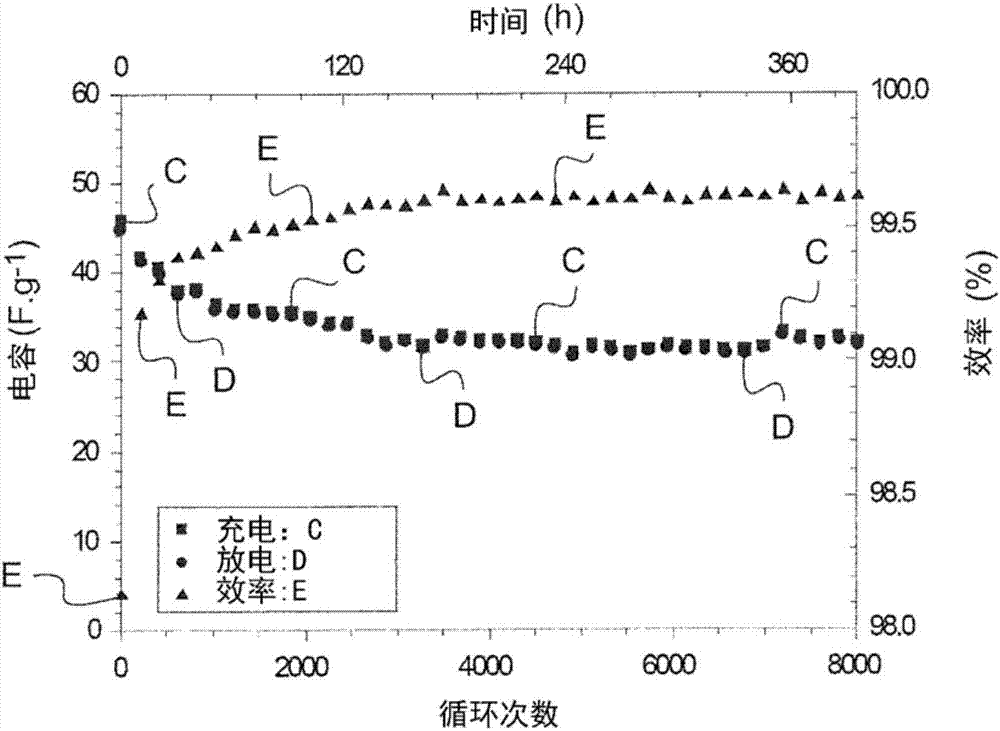
Embodiment 2
[0095] "Comparative" Example 2: Production of two ionic liquids not according to the present invention with different mass fractions respectively ion gel membrane
[0096] a) First "comparative" example: production of 2 ] / EMIMTFSI=[75 / 25] / 90 ions gel :
[0097]92 mg of PLA (PLA4060 HMw-HD, mass Mw=130 kDa from Natureworks) was mixed with 0.5 mL of acetonitrile to obtain a PLA concentration of approximately 180 g / L. The solution was stirred until the polymer was completely dissolved for approximately 2 hours. Then 937 mg of EMimTFSI and 110 μL of TEOS were added. The solution was homogenized by magnetic stirring for 10 minutes, and then 150 μL of formic acid was added to achieve a molar ratio r=(AF) / (TEOS)≧8. The solution was stirred for 1 to 2 minutes.
[0098] It was then coated onto a PEN support previously cleaned with acetone. Set the coating speed to 5cm.s -1 , with a deposition height of 300 μm. The film was gelled and air dried for 24 hours, then heated a...
Embodiment 3
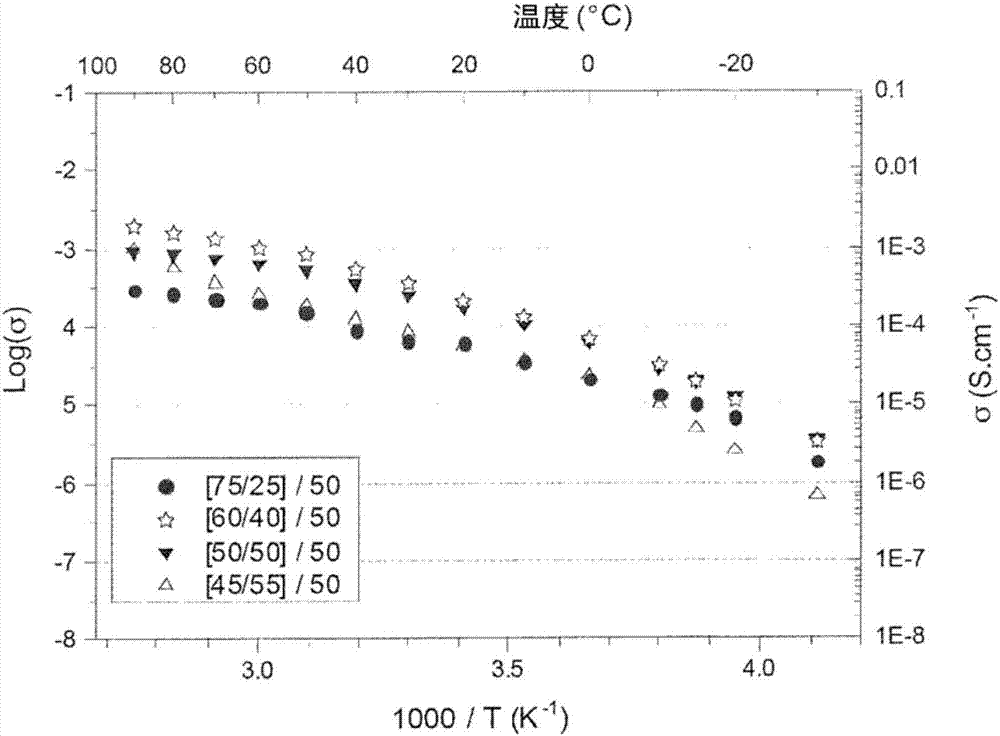
[0102] Embodiment 3: Production has the same ionic liquid mass fraction of 50% but has different [PLA / SiO 2 ]quality Comparison of four ion gel membranes according to the present invention
[0103] Compared to the "control" iongel 1 with composition [100 / 0] / 50 characterized by the absence of silicon condensation polymers, in EMIMTFSI containing 50 mass % but with four different [PLA / SiO 2 ] ratio between the four ion gels (see Figure 4-Figure 5 ). Each ion gel was prepared using PLA 4060HMw-HD from Natureworks and characterized by mass composition [PLA / SiO 2 ] / EMimTFSI, and prepared according to the following scheme:
[0104] - ion gel 2[75 / 25] / 50 : About 380 mg of PLA (Mw=130 kDa) was mixed with 2.2 mL of acetonitrile. The solution was stirred for about 2 hours. Then 507 mg of EMimTFSI and 462 μL of TEOS were added thereto. The solution was homogenized by magnetic stirring for 10 minutes, after which 648 μL of formic acid was added.
[0105] - ion gel 3[60 / 40]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com