Selective process for conversion of levulinic acid to gammavalerolactone
A technology of levulinic acid and valerolactone is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as unsatisfactory, and achieve the effect of short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Levulinic acid production involves the concept of a biorefinery, a facility that integrates biomass conversion methods and equipment to produce fuels, power and chemicals from biomass.
[0020] The chemical composition of biomass strongly depends on its source. The most abundant carbohydrate in nature is cellulose. Cellulose is an unbranched, water-insoluble polysaccharide composed of several hundred to up to tens of thousands of glucose units. Levulinic acid can be produced from a hexose sugar, such as glucose, by an acid-catalyzed reaction that yields 1 mole of both levulinic acid and formic acid from 1 mole of hexose sugar. Levulinic acid can be used as such for different applications, or further reacted to generate other bioprecursors or bioproducts.
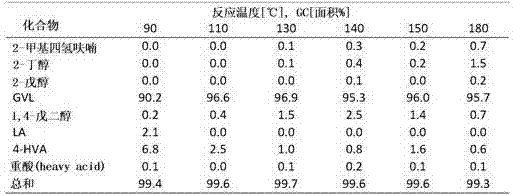
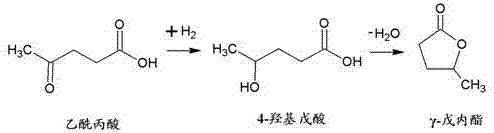
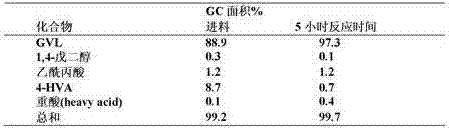
[0021] The reaction of levulinic acid to γ-valerolactone via 4-hydroxyvaleric acid was performed according to the following scheme.
[0022]
[0023] Although hydrogenation and cyclization are given as subsequen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com